
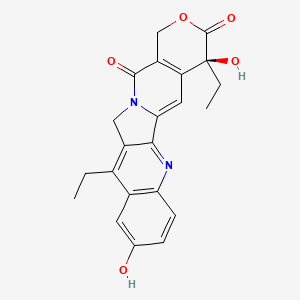

1. 7 Ethyl 10 Hydroxycamptothecin
2. Camptosar
3. Camptothecin 11
4. Camptothecin-11
5. Cpt 11
6. Cpt-11
7. Cpt11
8. Irinotecan
9. Irinotecan Hydrochloride
10. Irrinotecan
11. Nk012 Compound
12. Sn 38
13. Sn 38 11
14. Sn-38
15. Sn-38-11
16. Sn3811
1. 86639-52-3
2. Sn-38
3. Sn 38
4. Sn 38 Lactone
5. (s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
6. 10-hydroxy-7-ethylcamptothecin
7. Nk 012
8. Sn38
9. 7-ethyl-10-hydroxy-20(s)-camptothecin
10. Nk012
11. Nk-012
12. Irinotecan Related Compound B
13. Chebi:8988
14. 113015-38-6
15. It-141
16. Nsc673596
17. 0h43101t0j
18. (4s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
19. (+)-7-ethyl-10-hydroxycamptothecin
20. (19s)-10,19-diethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione
21. (4s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione
22. 7-ethyl-10-hydroxy-20(s)-campthothecin
23. Le-sn38
24. Captothecin, 7-ethyl-10-hydroxy-
25. Mfcd00871873
26. Unii-0h43101t0j
27. 110714-48-2
28. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4,11-diethyl-4,9-dihydroxy-, (4s)-
29. 1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, 4,11-diethyl-4,9-dihydroxy-, (4s)-
30. Avachem1025
31. Diethyl(dihydroxy)[?]dione
32. Sn 38- Bio-x
33. Schembl34018
34. Gtpl6925
35. Sn 38 [who-dd]
36. Dtxsid4040399
37. 10-hydroxy-7-ethyl Camptothecin
38. 10-hydroxy-7-ethyl-camptothecin
39. 7-ethyl-10-hydroxy Campthotecin
40. Ex-a989
41. Hms3413b12
42. Hms3652p12
43. Hms3677b12
44. Bcp01386
45. Zinc4099013
46. 7-ethyl-10-hydroxy-20(s)-cpt
47. Bdbm50418088
48. S4908
49. Akos015920433
50. Sn-38(nk-012)
51. Ac-1357
52. Bcp9000200
53. Ccg-264764
54. Cs-1579
55. Db05482
56. Nsc-673596
57. Ncgc00167831-01
58. Ncgc00167831-05
59. (4s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)dione
60. As-13533
61. Be164132
62. Bp-24513
63. Hy-13704
64. Nci60_026056
65. Camptothecin, 7-ethyl-10-hydroxy-
66. E0748
67. N2133
68. Sw219948-1
69. S-(+)-7-ethyl-10-hydroxycampothecin
70. Irinotecan Related Compound B [usp-rs]
71. 439e812
72. A857464
73. Q-100871
74. Q1750127
75. 7-ethyl-10-hydroxycamptothecin, >=98% (hplc), Powder
76. Irinotecan Related Compound B, United States Pharmacopeia (usp) Reference Standard
77. (19s)-10,19-diethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione
78. (4s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)dione, Aldrichcpr
79. (4s)-4,9-dihydroxy-4,11-diethyl-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione;sn-38
80. (s)-4,11-diethyl-4,9-di-oh-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
81. 1h-pyrano[3',7]indolizino[1,2-b]quinoline- 3,14(4h,12h)-dione, 4,11-diethyl-4,9-dihydroxy-, (4s)-
82. 7-ethyl-10-hydroxycamptothecin ((s)-4,11-diethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione)
83. H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4,11-diethyl-4,9-dihydroxy-, (s)-
84. Rs4
1. Sn-38
| Molecular Weight | 392.4 g/mol |
|---|---|
| Molecular Formula | C22H20N2O5 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 392.13722174 g/mol |
| Monoisotopic Mass | 392.13722174 g/mol |
| Topological Polar Surface Area | 100 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 820 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in colorectal cancer.
SN-38 (7-ethyl-10-hydroxycamptothecin) is the active metabolite of Irinotecan (CPT-11). Irinotecan is a topoisomerase I inhibitor commercially available as Camptosar. SN-38 has been found to be 2002000 times more cytotoxic than CPT-11, but has not been used as an anticancer drug due to its poor solubility in pharmaceutically acceptable solvents and low affinity to lipid membranes. SN-38 also undergoes a reversible conversion to an inactive open lactone ring structure at physiological pH. LE-SN-38 is a novel lipsome based formulation containing liposomes of uniform size distribution (<200 nm). Drug entrapment efficiency of the formulation is>95%.
Topoisomerase I Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE I. (See all compounds classified as Topoisomerase I Inhibitors.)
The entrapment of SN-38 in lipsomes results in a more stable and more soluble form of the drug. This allows for increased affinity of SN-38 to lipid membranes and improved delivery of the drug to tumor sites. SN-38 is a highly effective cytotoxic topoisomerase I inhibitor.
BUILDING BLOCK

MARKET PLACE

Reply
31 Jul 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
