31 May 2023
// PRESS RELEASE
23 Feb 2022
// PRESS RELEASE
09 Sep 2017
// INTEREM PRESS
Latest Content by PharmaCompass

 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES

![]() Reset all filters
Reset all filters
01 1Azithromycin
02 1Bepotastine
03 1Bimatoprost
04 2Brinzolamide
05 1Carboprost Tromethamine
06 1Dorzolamide Hydrochloride
07 1Etofenamate
08 1Febuxostat
09 1Latanoprost
10 1Latanoprostene Bunod
11 1Lifitegrast
12 1Nitazoxanide
13 1Pirisudanol
14 1Silodosin
15 1Tafluprost
16 1Travoprost

![]() Reset all filters
Reset all filters
01 3Active
02 14Blank
01 2Valid
02 15Blank

![]() Reset all filters
Reset all filters
01 1228MF10134
02 16Blank

![]() Reset all filters
Reset all filters
01 17Blank

![]() Reset all filters
Reset all filters
01 120220728-209-J-1261
02 16Blank

![]() Reset all filters
Reset all filters
01 17Blank
01 17Blank

 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32202
Submission : 2018-02-27
Status : Active
Type : II

Registration Number : 228MF10134
Registrant's Address : Avda. Mare de Deu de Montserrat 93-99, Pol. Ind. Sant Pere Molanta, 08799 Olerdola (Barcelona), Spain
Initial Date of Registration : 2016-07-25
Latest Date of Registration :

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-10-15
Pay. Date : 2021-06-29
DMF Number : 34369
Submission : 2020-01-27
Status : Active
Type : II

Certificate Number : R0-CEP 2021-059 - Rev 00
Issue Date : 2023-07-26
Type : Chemical
Substance Number : 2230
Status : Valid

| Available Reg. Filing : ASMF |

Certificate Number : CEP 2014-154 - Rev 01
Issue Date : 2024-02-27
Type : Chemical
Substance Number : 1513
Status : Valid

| Available Reg. Filing : ASMF |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34370
Submission : 2020-01-27
Status : Active
Type : II

| Available Reg. Filing : ASMF |

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2022-07-28
Registration Number : 20220728-209-J-1261
Manufacturer Name : Duke Chem, SA
Manufacturer Address : Pol. Industrial Sant Pere Molanta, Avgda. Mare de Déu de Montserrat, 93-99, Olèrdola, 08799 Barcelona, SPAIN
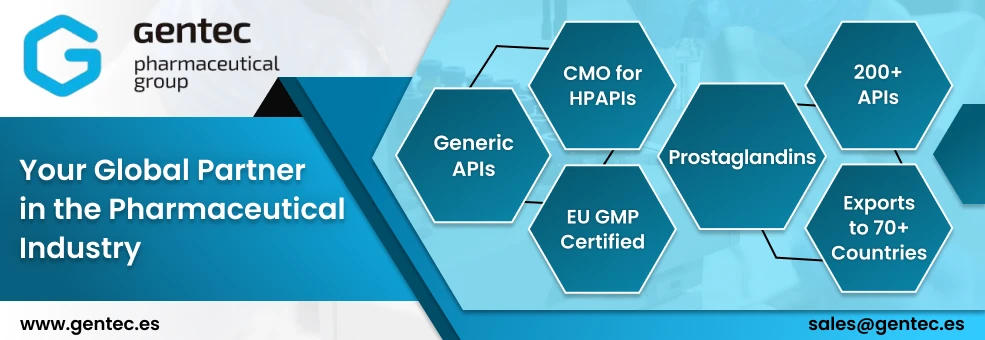
Gentec Pharmaceutical Group is a supplier offers 16 products (APIs, Excipients or Intermediates).
Find a price of Brinzolamide bulk with DMF, JDMF offered by Gentec Pharmaceutical Group
Find a price of Latanoprost bulk with DMF, CEP offered by Gentec Pharmaceutical Group
Find a price of Etofenamate bulk with CEP offered by Gentec Pharmaceutical Group
Find a price of Travoprost bulk with DMF offered by Gentec Pharmaceutical Group
Find a price of Azithromycin bulk offered by Gentec Pharmaceutical Group
Find a price of Bepotastine bulk offered by Gentec Pharmaceutical Group
Find a price of Bimatoprost bulk offered by Gentec Pharmaceutical Group
Find a price of Carboprost Tromethamine bulk offered by Gentec Pharmaceutical Group
Find a price of Dorzolamide Hydrochloride bulk offered by Gentec Pharmaceutical Group
Find a price of Febuxostat bulk offered by Gentec Pharmaceutical Group
Find a price of Latanoprostene Bunod bulk offered by Gentec Pharmaceutical Group
Find a price of Lifitegrast bulk offered by Gentec Pharmaceutical Group
Find a price of Nitazoxanide bulk offered by Gentec Pharmaceutical Group
Find a price of Pirisudanol bulk offered by Gentec Pharmaceutical Group
Find a price of Silodosin bulk offered by Gentec Pharmaceutical Group
Find a price of Tafluprost bulk offered by Gentec Pharmaceutical Group