NDC Code(s) : 66689-401-01, 66689-401-50, 66689-403-16
Packager : VistaPharm, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Oxycodone HydrochlorideOxycodone Hydrochloride SOLUTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Oxycodone HydrochlorideOxycodone Hydrochloride SOLUTION | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - VistaPharm, Inc.(116743084) |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mL Cup Label
Oxycodone
Hydrochloride Oral Solution, USP CII
5 mg per 5 mL
(1 mg/mL)
PHARMACIST: Dispense the enclosed Medication Guide to each patient.
Delivers 5 mL
Store at 20°-25°C (68°-77°F); see USP CRT conditions.
XACTDOSE
Manufactured by:
VistaPharm®
Largo, FL 33771, USA
Rx Only
VP2012R3
06/18
NDC 66689-401-01

PRINCIPAL DISPLAY PANEL
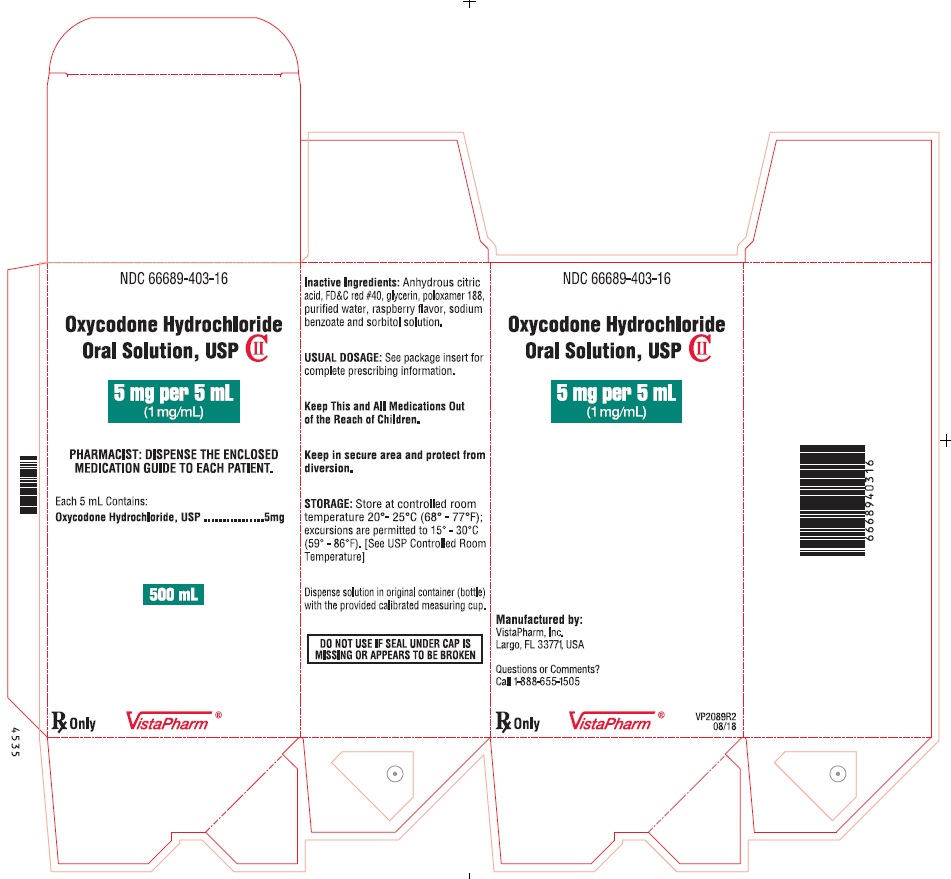
NDC 66689-403-16
Oxycodone Hydrochloride Oral Solution, USP CII
5 mg per 5 mL
(1 mg/mL)
PHARMACIST: DISPENSE THE ACCOMPANYING MEDICATION GUIDE TO EACH PATIENT.
Each 5 mL Contains:
Oxycodone Hydrochloride, USP ........................................... 5 mg
DO NOT USE IF SEAL UNDER CAP IS MISSING OR BROKEN.
USUAL DOSAGE: See package insert for complete prescribing information.
STORAGE: Store at controlled room temperature 20°- 25°C (68° - 77°F); excursions are permitted to 15° - 30°C (59° - 86°F). [See USP Controlled Room Temperature]
FOR ORAL USE ONLY
500 mL
Dispense solution in original container (bottle) with the provided calibrated measuring cup.
Keep in secured area and protect from diversion.
Manufactured by: VistaPharm, Inc., Largo, FL 33771, USA
Rx Only
VistaPharm
VP2011R3
08/18

PRINCIPAL DISPLAY PANEL
NDC 66689-403-16
Oxycodone Hydrochloride Oral Solution, USP CII
5 mg per 5 mL
(1 mg/mL)
PHARMACIST: DISPENSE THE ENCLOSED MEDICATION GUIDE TO EACH PATIENT.
Each 5 mL Contains:
Oxycodone Hydrochloride, USP..............5 mg
500 mL
Rx Only
VistaPharm