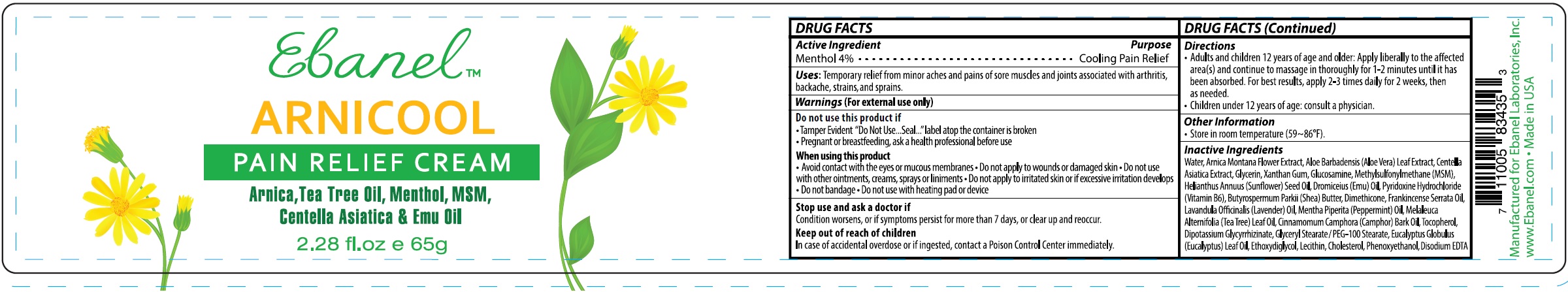
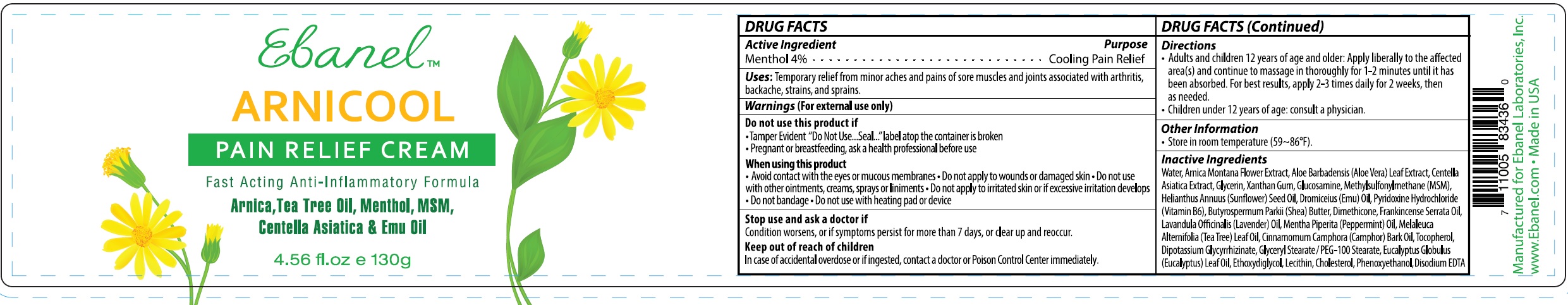
NDC Code(s) : 63742-007-00, 63742-007-01
Packager : Clinical Resolution Laboratory, Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ARNICOOLMENTHOL CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Clinical Resolution Laboratory, Inc.(825047942) |
PRINCIPAL DISPLAY PANEL