NDC Code(s) : 0641-6166-01, 0641-6166-10, 0641-6167-01, 0641-6167-10
Packager : Hikma Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Amikacin SulfateAmikacin Sulfate INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Amikacin SulfateAmikacin Sulfate INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Hikma Pharmaceuticals USA Inc.(946499746) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Hikma Pharmaceuticals USA Inc. | 946499746 | analysis(0641-6166, 0641-6167), label(0641-6166, 0641-6167), manufacture(0641-6166, 0641-6167), pack(0641-6166, 0641-6167) | |
PRINCIPAL DISPLAY PANEL
NDC 0641-6167-01 Rx only
Amikacin
Sulfate
Injection, USP
equivalent to amikacin
500 mg per 2 mL
(250 mg/mL)
For IM or IV use
2 mL Single Dose Vial

NDC 0641-6167-10 Rx only
Amikacin
Sulfate Injection, USP
equivalent to amikacin
500 mg per 2 mL
(250 mg/mL)
For Intramuscular or
Intravenous use
10 x 2 mL Single Dose Vials

PRINCIPAL DISPLAY PANEL
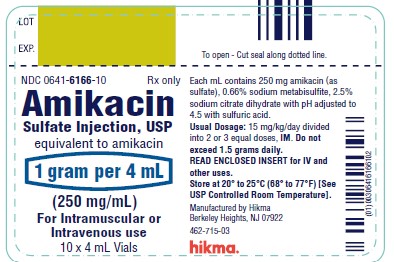
NDC 0641-6166-01 Rx only
Amikacin
Sulfate Injection, USP
equivalent to amikacin
1 g per 4 mL
(250 mg/mL)
For IM or IV use
4 mL Vial

NDC 0641-6166-10 Rx only
Amikacin
Sulfate Injection, USP
equivalent to amikacin
1 g per 4 mL
(250 mg/mL)
For Intramuscular or Intravenous use
10 x 4 mL Vials