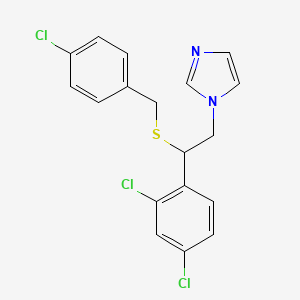

1. 1-(beta-(4''-chlorobenzylthio)-2'4'-dichlorophenethyl)imidazole
2. Exelderm
3. Myk
4. Sulconazole Mononitrate
5. Sulconazole Nitrate
6. Sulconazole, Mononitrate, (+-)-isomer
1. 61318-90-9
2. Sulconazolum
3. Sulconazol
4. Sulconazolum [inn-latin]
5. 1-(2-((4-chlorobenzyl)thio)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
6. Sulconazol [inn-spanish]
7. (+/-)-sulconazole
8. 1-[2-[(4-chlorophenyl)methylsulfanyl]-2-(2,4-dichlorophenyl)ethyl]imidazole
9. 5d9haa5q5s
10. Chebi:77776
11. Sulconazole (inn)
12. 1-{2-[(4-chlorobenzyl)sulfanyl]-2-(2,4-dichlorophenyl)ethyl}-1h-imidazole
13. Sulconazole [inn]
14. 1-[2-(4-chloro-benzylsulfanyl)-2-(2,4-dichloro-phenyl)-ethyl]-1h-imidazole
15. Sulconazole [inn:ban]
16. Unii-5d9haa5q5s
17. 1h-imidazole, 1-(2-(((4-chlorophenyl)methyl)thio)-2-(2,4-dichlorophenyl)ethyl)-
18. 1h-imidazole, 1-[2-[[(4-chlorophenyl)methyl]thio]-2-(2,4-dichlorophenyl)ethyl]-
19. Spectrum_001416
20. Sulconazole [mi]
21. Prestwick0_000810
22. Prestwick1_000810
23. Prestwick2_000810
24. Prestwick3_000810
25. Spectrum2_001422
26. Spectrum3_001457
27. Spectrum4_000436
28. Spectrum5_001155
29. Sulconazole [vandf]
30. Chembl1221
31. Schembl34761
32. Bspbio_000679
33. Bspbio_002953
34. Kbiogr_000792
35. Kbioss_001896
36. Sulconazole [who-dd]
37. Cid_65495
38. Divk1c_000220
39. Spbio_001524
40. Spbio_002600
41. Bpbio1_000747
42. Dtxsid8044129
43. Bdbm31770
44. Hy-b1460b
45. Kbio1_000220
46. Kbio2_001896
47. Kbio2_004464
48. Kbio2_007032
49. Kbio3_002453
50. Ninds_000220
51. (+-)-1-(2,4-dichlor-beta-((4-chlorbenzyl)thio)phenethyl)imidazol
52. (+-)-1-(2,4-dichloro-beta-((p-chlorobenzyl)thio)phenethyl)imidazole
53. Akos015961204
54. Db06820
55. 1h-imidazole, 1-(2-(((4-chlorophenyl)methyl)thio)-2-(2,4-dichlorophenyl)ethyl)-, (+-)-
56. Idi1_000220
57. Ncgc00018250-02
58. Ac-13116
59. Sbi-0051660.p002
60. Db-053840
61. Ab00053607
62. Cs-0013811
63. Ft-0630725
64. C08076
65. D08535
66. Ab00053607_16
67. Ab00053607_17
68. Q2392530
69. Brd-a70649075-008-05-0
70. Brd-a70649075-008-17-5
71. 1-[2,4-dichloro-beta-(4-chlorobenzylthio)phenethyl]imidazole
72. 1-[beta-[(4-chlorobenzyl)thio]-2,4-dichlorophenethyl]-1h-imidazole
73. 1-(2-(4-chlorobenzylthio)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
74. 1-(2-(((4-chlorophenyl)methyl)thio)-2-(2,4-dichlorophenyl)ethyl)-1h-imidazole
75. 1h-imidazole, 1-[2-[[(4-chlorophenyl)methyl]thio]-2-(2,4-dichlorophenyl)ethyl]-(9ci)
76. 1h-imidazole, 1-(2-(((4-chlorophenyl)methyl)thio)-2-(2,4-dichlorophenyl)ethyl)-, (+/-)-
| Molecular Weight | 397.7 g/mol |
|---|---|
| Molecular Formula | C18H15Cl3N2S |
| XLogP3 | 6.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 396.002153 g/mol |
| Monoisotopic Mass | 396.002153 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 379 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sulconazole solution 1.0% is indicated for the treatment of tinea cruris and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; and for the treatment of tinea versicolor. Effectiveness has not been proven in tinea pedis (athletes foot).
FDA Label
The function of imidazole derivatives can be attributed to their structural resemblance to purines essential to metabolism.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D01AC09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AC - Imidazole and triazole derivatives
D01AC09 - Sulconazole
Absorption
Total sulconazole systemic absorption after topical administration was ~8.71% of the dose.
Route of Elimination
About 6.70% of the dose was recovered in urine, and 2.01% in feces over a 7 day collection period. Radioactivity could be detected in both urine and feces at 7 days potentially due to a reservoir effect.