

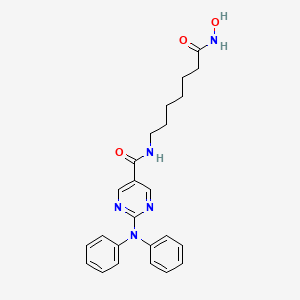

1. 2-(diphenylamino)-n-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide
2. Acy-1215
3. Rocilinostat
1. 1316214-52-4
2. Acy-1215
3. Rocilinostat
4. 2-(diphenylamino)-n-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide
5. Rocilinostat (acy-1215)
6. Acy-63
7. Ricolinostat (acy-1215)
8. N-[7-(hydroxyamino)-7-oxoheptyl]-2-(n-phenylanilino)pyrimidine-5-carboxamide
9. Wkt909c62b
10. 7-{[2-(diphenylamino)pyrimidin-5-yl]formamido}-n-hydroxyheptanamide
11. Mfcd22666356
12. 2-(diphenylamino)-n-[7-(hydroxyamino)-7-oxoheptyl]pyrimidine-5-carboxamide
13. 5-pyrimidinecarboxamide, 2-(diphenylamino)-n-(7-(hydroxyamino)-7-oxoheptyl)-
14. Ricolinostat [usan]
15. Ricolinostat [usan:inn]
16. Unii-wkt909c62b
17. 5-pyrimidinecarboxamide, 2-(diphenylamino)-n-[7-(hydroxyamino)-7-oxoheptyl]-
18. Acy1215
19. Ricolinostat (usan/inn)
20. Ricolinostat [inn]
21. 2-(diphenylamino)-n-[7-(hydroxyamino)-7-oxoheptyl]-5-pyrimidinecarboxamide
22. Mls006011181
23. Ricolinostat [who-dd]
24. Schembl574580
25. Gtpl7010
26. Chembl2364628
27. Chebi:95073
28. Dtxsid40157148
29. Ex-a139
30. Hms3426c09
31. Hms3653f17
32. Hms3886l21
33. Acy 1215
34. Amy38185
35. Bcp06028
36. Bdbm50439674
37. Nsc767952
38. S8001
39. Zinc89630354
40. Akos024259260
41. Ccg-269054
42. Cs-0965
43. Db12376
44. Nsc-767952
45. Sb17054
46. Ncgc00345802-01
47. Ncgc00345802-05
48. Ac-30258
49. As-73344
50. Ba164806
51. Hy-16026
52. Smr004702950
53. Sy096614
54. Sw219836-1
55. D10661
56. J-690127
57. Q27088553
58. Ah4
59. Us8609678, 2-(diphenylamino)-n-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide [26]
| Molecular Weight | 433.5 g/mol |
|---|---|
| Molecular Formula | C24H27N5O3 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Exact Mass | 433.21138974 g/mol |
| Monoisotopic Mass | 433.21138974 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 538 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Histone Deacetylase Inhibitors
Compounds that inhibit HISTONE DEACETYLASES. This class of drugs may influence gene expression by increasing the level of acetylated HISTONES in specific CHROMATIN domains. (See all compounds classified as Histone Deacetylase Inhibitors.)
