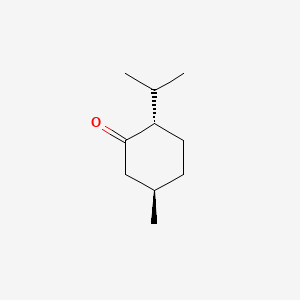

1. (-)-menthone
2. 14073-97-3
3. L-menthone
4. Trans-menthone
5. (2s,5r)-2-isopropyl-5-methylcyclohexanone
6. P-menthone
7. L-menthan-3-one
8. Neomenthone
9. Menthone Racemic
10. Trans-menthan-3-one
11. 89-80-5
12. Trans-p-menthan-3-one
13. (1r,4s)-(-)-p-menthan-3-one
14. Cyclohexanone, 5-methyl-2-(1-methylethyl)-, (2s,5r)-
15. Dl-menthone
16. P-menthan-3-one, Trans-
17. Fema No. 2667
18. (2s,5r)-5-methyl-2-(propan-2-yl)cyclohexanone
19. (-)-(2s,5r)-menthone
20. (1r,4s)-p-menthan-3-one
21. (2s,5r)-5-methyl-2-(1-methylethyl)cyclohexanone
22. (2s,5r)-5-methyl-2-propan-2-ylcyclohexan-1-one
23. (2s-trans)-5-methyl-2-(1-methylethyl)cyclohexanone
24. Cyclohexanone, 5-methyl-2-(1-methylethyl)-, Trans-
25. (-)-(1r,4s)-menthone
26. (2s,5r)-5-methyl-2-(1-methylethyl)-cyclohexanone
27. Chebi:15410
28. (-)-5-methyl-2-(1-methylethyl)cyclohexanone
29. 5f709w4og4
30. Cyclohexanone, 5-methyl-2-(1-methylethyl)-, (2s-trans)-
31. Dsstox_cid_24384
32. Dsstox_rid_80188
33. Dsstox_gsid_44384
34. Menthone (natural)
35. P-menthan-3-one Racemic
36. L-p-menthan-3-one
37. (dl)-menthone
38. 5-methyl-2-(1-methylethyl)cyclohexanone
39. Cas-14073-97-3
40. Ccris 5747
41. Hsdb 1268
42. P-menthan-3-one, Dl-
43. Einecs 201-941-1
44. Einecs 214-049-2
45. Unii-9nh5j4v8fn
46. Menthon
47. Unii-5f709w4og4
48. Ai3-11106
49. Menthone G
50. Trans-p-menthone
51. 1-menthone
52. (-)menthone
53. 5-methyl-2-(1-methylethyl)cyclohexanone, Trans-
54. Ncgc00095606-01
55. Cyclohexan-1-one, 2-isopropyl-5-methyl-, Racemic
56. Einecs 237-926-1
57. Mfcd00001634
58. Menthan-3-one, Trans
59. Spectrum_001299
60. Rel-(2r,5s)-2-isopropyl-5-methylcyclohexanone
61. (-)-menthone,(s)
62. Menthone [fhfi]
63. Specplus_000437
64. Menthone, L-
65. L-menthone [mi]
66. Spectrum2_000691
67. Spectrum3_001272
68. Spectrum4_001648
69. Spectrum5_000495
70. (2s,5r)-2-isopropyl-5-methyl-cyclohexanone
71. Bmse000375
72. (-)-menthone, 90%
73. Ec 237-926-1
74. 9nh5j4v8fn
75. Schembl21709
76. Bspbio_002864
77. Kbiogr_002115
78. Kbioss_001779
79. Menthone, (-)-
80. Spectrum300564
81. Divk1c_006533
82. Spbio_000841
83. (1s)-trans-p-menthan-3-one
84. (-)-menthone [fcc]
85. (2s,5r)-5-methyl-2-(propan-2-yl)cyclohexan-1-one
86. Chembl276311
87. Dtxsid2044478
88. Dtxsid3044384
89. Kbio1_001477
90. Kbio2_001779
91. Kbio2_004347
92. Kbio2_006915
93. Kbio3_002364
94. Cyclohexanone, 5-methyl-2-(1-methylethyl)-, Trans-(+/-)-
95. Zinc967796
96. (-)-menthone, Analytical Standard
97. Hy-n7916
98. Tox21_111510
99. Tox21_302153
100. Ccg-38562
101. Mfcd00136033
102. Akos006343213
103. Tox21_111510_1
104. Lmpr0102090004
105. Sdccgmls-0066582.p001
106. 2-isopropyl-5-methylcyclohexanone, Trans
107. Ncgc00095606-02
108. Ncgc00178425-01
109. Ncgc00255957-01
110. As-17440
111. (2s,5r)2-isopropyl-5-methylcyclohexanone
112. Cs-0138798
113. 1-methyl-4-isopropylcyclohexan-3-one
114. C00843
115. (2s,5r)-2-isopropyl-5-methylcyclohexan-1-one
116. (2s, 5r)-trans-2-isopropyl-5-methylcyclohexanone
117. Q424902
118. Sr-05000002387
119. 5-methyl-2-(1-methylethyl)-(2s,5r)-cyclohexanone
120. L-menthone, Mixture Of Isomers, >=96%, Fcc, Fg
121. Sr-05000002387-1
122. W-108194
123. 5-methyl-2-(1-methylethyl)-(2s-trans)-cyclohexanone
124. (-)-menthone, Primary Pharmaceutical Reference Standard
125. Cyclohexanone, 5-methyl-2-(1-methylethyl)-, Trans-(.+/-.)-
126. 1/c10h18o/c1-7(2)9-5-4-8(3)6-10(9)11/h7-9h,4-6h2,1-3h3/t8-,9+/m1/s
| Molecular Weight | 154.25 g/mol |
|---|---|
| Molecular Formula | C10H18O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 154.135765193 g/mol |
| Monoisotopic Mass | 154.135765193 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 149 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...KETONES (EG CARVONE & MENTHONE) ARE REDUCED TO SECONDARY ALCOHOLS WHICH ARE THEN EXCRETED AS GLUCURONIDES. MENTHONE, ASYMMETRIC REDUCTION /TO/ NEO-MENTHOL, GLUCURONIDE CONJUGATION /TO/ MENTHYL GLUCURONIDE.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 149