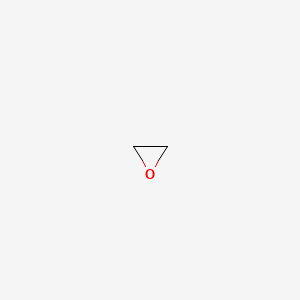

1. Oxide, Ethylene
2. Oxirane
1. Oxirane
2. Epoxyethane
3. 75-21-8
4. 1,2-epoxyethane
5. Oxacyclopropane
6. Dihydrooxirene
7. Oxidoethane
8. Oxyfume
9. Ethene Oxide
10. Dimethylene Oxide
11. Amprolene
12. Anprolene
13. Anproline
14. Aethylenoxid
15. 1,2-epoxyaethan
16. Merpol
17. Oxiran
18. Oxyfume 12
19. T-gas
20. Oxirene, Dihydro-
21. Fema No. 2433
22. Ethyleenoxide
23. Oxiraan
24. Etylenu Tlenek
25. Qazi-ketcham
26. Oxyde D'ethylene
27. Rcra Waste Number U115
28. Eto
29. Nci-c50088
30. Ent-26263
31. Un 1040
32. Chebi:27561
33. Jjh7gnn18p
34. E.o.
35. Epoxide Or Oxirane
36. Oxiraan [dutch]
37. Aethylenoxid [german]
38. Caswell No. 443
39. Ethyleenoxide [dutch]
40. Ethyleneoxide
41. Ethylenoxide
42. Ethox
43. Etylenu Tlenek [polish]
44. 1,2-epoxyaethan [german]
45. Ccris 297
46. Etilene (ossido Di)
47. Hsdb 170
48. Ethylene (oxyde D') [french]
49. Etilene (ossido Di) [italian]
50. Alpha,beta-oxidoethane
51. Ethylene (oxyde D')
52. Einecs 200-849-9
53. Unii-jjh7gnn18p
54. Un1040
55. Rcra Waste No. U115
56. Epa Pesticide Chemical Code 042301
57. Ethyleneoxy
58. Monooxirane
59. Ethylene-oxide
60. Epoxy Ethane
61. Ai3-26263
62. Epitope Id:116215
63. Ec 200-849-9
64. .alpha.,.beta.-oxidoethane
65. Ciba-geigy 9138
66. Ethylene Oxide [mi]
67. 11104-97-5
68. Ethylene Oxide [fhfi]
69. Ethylene Oxide [hsdb]
70. Ethylene Oxide [iarc]
71. Ethylene Oxide, >=99.5%
72. Ethylene Oxide, >=99.9%
73. Ethylene Oxide [mart.]
74. Chembl1743219
75. Dtxsid0020600
76. Ethylene Oxide (30% Or Less), Propylene Oxide Mixture
77. Ethylene Oxide [who-dd]
78. Ethylene Oxide, Purum, >=99.8%
79. C0527
80. Mfcd00014482
81. Sterilizing Gas Ethylene Oxide 100%
82. Akos009031564
83. E0647
84. E0689
85. E0690
86. E0692
87. E0693
88. Ethylene Oxide 1000 Microg/ml In Triacetin
89. Ethylene Oxide Solution, 2.5-3.3 M In Thf
90. C06548
91. D03474
92. Q407473
93. Ethylene Oxide Solution, Certified Reference Material, 50 Mg/ml In Methanol
94. Ethylene Oxide Solution, 50 Mg/ml In Methylene Chloride, Analytical Standard
95. Ethylene Oxide Solution, Certified Reference Material, 500 Mug/ml In Dmso, Ampule Of 1 Ml
96. Ethylene Oxide Solution, Certified Reference Material, 2000 Mug/ml In Dichloromethane, Ampule Of 1 Ml
97. Ethylene Oxide, Or Ethlene Oxide With Nitrogen Up To A Total Pressure Of 1mpa (10 Bar) At 50 Degrees C
98. Ethylene Oxide, Or Ethlene Oxide With Nitrogen Up To A Total Pressure Of 1mpa (10 Bar) At 50 Degrees C [un1040] [poison Gas]
| Molecular Weight | 44.05 g/mol |
|---|---|
| Molecular Formula | C2H4O |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 44.026214747 g/mol |
| Monoisotopic Mass | 44.026214747 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 10.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
The study reported here examined the dosimetry of ethylene oxide (EO) in male B6C3F1 mice by direct determination of blood EO concentrations. Steady-state blood EO concentrations were measured during a single 4-hr nose-only inhalation exposure (0, 50, 100, 200, 300, or 400 ppm EO). In addition, glutathione (GSH) concentrations were measured in liver, lung, kidney, and testis to assess the role of the GSH depletion in the saturable metabolism previously observed in mice. Blood EO concentrations were found to increase linearly with exposure concentration up to 200 ppm. Markedly sublinear blood dosimetry was observed at exposure concentrations exceeding 200 ppm. An EO exposure concentration-dependent reduction in tissue GSH levels was observed, with both liver and lung GSH levels significantly depressed at EO exposure concentrations of 100 ppm or greater. /The/ results also indicate that depletion of GSH is likely responsible for nonlinear dosimetry of EO in mice and that GSH depletion corresponds with reports of dose-rate effects in mice exposed to EO.
PMID:9473528 Brown CD et al; Toxicol Appl Pharmacol 148 (2): 215-21 (1998)
... The objectives of this study were to examine the relationship between cigarette smoking and hemoglobin adducts derived from ... EO and to investigate whether null genotypes for glutathione transferase (GSTM1 and GSTT1) alter the internal dose of these agents. The hemoglobin adduct ... N-(2-hydroxyethyl)valine (HEVal), which is formed from EO, and GST genotypes were determined in blood samples obtained from 16 nonsmokers and 32 smokers (one to two packs/day). Smoking information was obtained by questionnaire, and plasma cotinine levels were determined by immunoassay. Glutathione transferase null genotypes (GSTM1 and GSTT1) were determined by PCR. ... HEVal levels increased with increased cigarette smoking dose (both self-reported and cotinine-based). ... HEVal levels were also correlated. ... GSTM1 null genotypes had no significant impact on HEVal. However, HEVal levels were significantly elevated in GSTT1-null individuals when normalized to smoking status or cotinine levels. ... The lack of a functional GSTT1 is estimated to increase the internal dose of EO derived from cigarette smoke by 50-70%.
PMID:10919741 Fennell TR et al; Cancer Epidemiol Biomarkers Prev 9 (7): 705-12 (2000)
AFTER EXPOSURE OF MICE TO MIXT OF 1,2-(3)H-ETHYLENE OXIDE VAPOR IN AIR FOR 75 MIN, 90-95% OF RADIOACTIVITY WAS ELIMINATED IN 24 HR. HIGHEST CONCN OF RESIDUAL RADIOACTIVITY WERE FOUND IN PROTEIN FRACTIONS OF SPLEEN; SMALLER AMT OCCURRED IN LIVER, KIDNEY, LUNG & TESTIS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V11 162 (1976)
Iv injection of (14)C-labeled ethylene oxide indicated that (14)C concn in the testicle, epididymis and other organs were higher than those in the blood when measured 20 min to 4 hr after exposure. Radioactivity was still present in the epididymis 24 hr after exposure had ended.
Appelgren LE et al; Europ Soc Toxicol 18: 315 (1977)
For more Absorption, Distribution and Excretion (Complete) data for ETHYLENE OXIDE (11 total), please visit the HSDB record page.
Ethylene oxide reacts with glutathione to form cysteine derivatives, forms ethylene glycol by epoxide hydrolase with subsequent metabolism of the glycol, and reacts with chloride to form 2-chloroethanol. The relative importance of these pathways is undefined. Ethylene glycol glutathione conjugates are metabolites of ethylene oxide. ...
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1244
In adult male Sprague-Dawley rats, male Swiss CD-1 mice, and male rabbits, 20 or 60 mg/kg ethylene oxide as a solution in distilled water was injected into the caudal vein in rats and mice or in the marginal vein in rabbits. Some animals were exposed to 200 ppm ethylene oxide in inhalation chambers. The animals were housed in metabolism cages, and urine samples were collected at 0-6 hr and 6-24 hr. The urine samples were analyzed for 2-hydroxyethylmercapturic acid, N-acetyl-S-carboxy-methyl-L cysteine, S-(2-hydroxyethyl)-L-cysteine, S-carboxymethyl-L-cysteine, and ethylene glycol. Species-related differences in the metabolic disposition of ethylene oxide were observed. Excretion product patterns did not differ significantly between injected doses. Rats (n= 5) eliminated 37% of ethylene oxide as 2-hydroxyethylmercapturic acid (31%) and ethylene glycol (6%); mice (n= 10) converted 19.3% of the ethylene oxide to 2-hydroxyethylmercapturic acid (8.3%), S-2-hydroxyethyl-L-cysteine (5.8%), S-carboxymethyl-L-cysteine (1.9%), and ethylene glycol (3.3%). The rabbits (n= 3) excreted only 2% of the ethylene oxide, primarily as ethylene glycol. In rats, larger amounts of 2-hydroxyethylmercapturic acid were excreted in the 6-24 hr period, and larger amounts of ethylene glycol were excreted in the 0-6 hr period. In mice, equal amounts of 3-hydroxyethylmercapturic acid were excreted in the two collection periods and larger amounts of ethylene glycol were excreted in the 6-24 hr period. No urine was voided by the rabbits in the 0-6 hr period. No qualitative differences in urinary metabolite excretion of ethylene oxide were observed relative to the method of exposure.
PMID:3692004 Tardif R et al; Fundam Appl Toxicol 9 (3): 448-53 (1987)
In the presence of water and chloride, ethylene oxide is hydrolyzed to 2-chloroethanol and ethylene glycol. The glycol is further metabolized to oxalate, formic acid and CO2.
European Chemicals Bureau; IUCLID Dataset, Ethylene Oxide (CAS # 75-21-8) p. 155 (2000 CD-ROM edition). Available from, as of July 9, 2008: https://esis.jrc.ec.europa.eu/
Within 24 hours following iv treatment 35% of the administered doses ranging from 1 to 10 mg/kg to the rat were excreted as 2-hydroxyethyl-mercapturic acid (2-HEMA) in the urine. After inhalation exposure to different ethylene oxide concentrations, the 2-HEMA levels determined in 24 hr-urine were linearly related to ethylene oxide exposure levels.
European Chemicals Bureau; IUCLID Dataset, Ethylene Oxide (CAS # 75-21-8) p. 155 (2000 CD-ROM edition). Available from, as of July 9, 2008: https://esis.jrc.ec.europa.eu/
In animals and humans, there are two routes of ethylene oxide catabolism, both of which are considered to be detoxification pathways. The first involves hydrolysis to ethylene glycol, with subsequent conversion to oxalic acid, formic acid, and carbon dioxide. The second involves conjugation with glutathione, with subsequent metabolic steps yielding S-(2-hydroxyethyl)cysteine (S-(2-carboxymethyl)cysteine) and N-acetylated derivatives (ie, N-acetyl-S-(2-hydroxyethyl)cysteine (and N-acetyl-S-(2-carboxymethyl)cysteine)) ... . The route involving conjugation with glutathione appears to predominate in rats and mice; in larger species (rabbits, dogs), the conversion of ethylene oxide is primarily via hydrolysis through ethylene glycol ... . Ethylene oxide may also be formed from the metabolism of ethylene ... . A physiologically based pharmacokinetic (PBPK) model for the dosimetry of inhaled ethylene oxide was first developed for rats and included binding of ethylene oxide to hemoglobin and DNA in addition to tissue distribution, metabolic pathways (ie, hydrolysis by epoxide hydrolase and conjugation by glutathione-S-transferase), and depletion of hepatic and extra-hepatic glutathione ... . The model was then refined and extended to mice and humans ... . Simulations indicate that in mice, rats, and humans, about 80%, 60%, and 20%, respectively, would be metabolized via glutathione conjugation ... . This is consistent with observed levels of theta-class glutathione S-transferase (GSTT1) enzyme activity in the order mice > rats > humans. In rats and mice, GSTT1 activity was highest in the liver, followed by the kidney and testes. Rat brain and rat and mouse lung contained small amounts of activity compared with other tissues (enzyme activity in mouse brain was not examined). Ethylene oxide is a substrate for the human GSTT1 enzyme ... .
International Programme on Chemical Safety; Concise International Chemical Assessment Document Number 54: ETHYLENE OXIDE (2003). Available from, as of July 22, 2008: https://www.inchem.org/pages/cicads.html
No reports found; [TDR, p. 694]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 694
Labeled ethylene oxide is primarily excreted in urine and a half-life of approximately 10 min was estimated for the first-order clearance of ethylene oxide in rodents.
USEPA/OPPTS; Revised Ethylene Oxide HED Risk Assessment for Reregistration Eligibility Document (RED) p.9 EPA-HQ-OPP-2005-0203-0100 (May 2007). Available from, as of July 21, 2008: https://www.regulations.gov/search/Regs/home.html#home
The elimination half life is estimated at 40 to 55 minutes in humans.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1244
Ethylene oxide is an electrophilic agent that alkylates nucleophilic groups in biological macromolecules .... Since ethylene oxide is formed during the metabolism of ethylene, a natural body constituent, endogenous as well as exogenous sources of ethylene and ethylene oxide contribute to background alkylation of proteins such as hemoglobin and albumin, as well as DNA ... . N-(2-Hydroxyethyl)valine (HEVal) and hydroxyethylhistidine (HEHis) adducts have been frequently monitored in tissues of workers exposed to ethylene oxide in occupational settings ... . Background levels of HEVal in non-smokers ranged from 9 to 188 pmol/g globin ... Studies of smokers exposed to ethylene oxide in cigarette smoke ... and occupationally exposed workers ... have revealed higher levels of hemoglobin HEVal adducts among individuals with a GSTT1 null genotype (ie, homozygous deletion of GSTT1 gene) than among those with a GSTT1 positive genotype (ie, having at least one copy of the GSTT1 gene). ...
International Programme on Chemical Safety; Concise International Chemical Assessment Document Number 54: ETHYLENE OXIDE (2003). Available from, as of July 22, 2008: https://www.inchem.org/pages/cicads.html
Ethylene oxide binding to DNA results primarily in the formation of 7-(2-hydroxyethyl)guanine (7-HEGua) ... . In DNA extracted from the lymphocytes of unexposed individuals, mean background levels of 7-HEGua ranged from 2 to 8.5 pmol/mg DNA ... Human tissue contains 10- to 15-fold higher levels of endogenous 7-HEGua than rodent tissue. ... In mice, half-lives for the removal of 7-HEGua in DNA from a variety of tissues (brain, lung, spleen, liver, and testes) were 1.5- to 3.9-fold lower than in rats ... . In both rats and mice, substantive depletion of glutathione pools has been observed following single exposure to high levels (ie, > 550 mg/cu m) of ethylene oxide ... although it should be noted that increases in tumour incidence have been observed at lower concentrations. ...
International Programme on Chemical Safety; Concise International Chemical Assessment Document Number 54: ETHYLENE OXIDE (2003). Available from, as of July 22, 2008: https://www.inchem.org/pages/cicads.html
The /physiologically based pharmacokinetic (PBPK)/ models for rats, mice, and humans are qualitatively similar in their elements and provide for interspecies comparisons of internal ethylene oxide dose. The models are consistent with the conclusion that ethylene oxide is acting as a direct-acting alkylating agent in humans and rodents. Quantitative differences in response in biomarkers of exposure and effect are accounted for by differences in basic physiology between rodents and humans, rather than by factors suggesting a different mode of action.
International Programme on Chemical Safety; Concise International Chemical Assessment Document Number 54: ETHYLENE OXIDE (2003). Available from, as of July 22, 2008: https://www.inchem.org/pages/cicads.html
Evidence for a common mechanism of carcinogenesis in humans and experimental animals comes from studies that have demonstrated similar genetic damage in cells of exposed animals and workers. The DNA damaging activity of ethylene oxide provides its effectiveness as a sterilant, and it is this same property that accounts for its carcinogenic risk to humans.
DHHS/NTP; SUBSTANCE PROFILES REPORT ON CARCINOGENS, ELEVENTH EDITION: Ethylene Oxide CAS No. 75-21-8 p.1. Available from, as of July 23, 2008: https://ntp.niehs.nih.gov/