
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
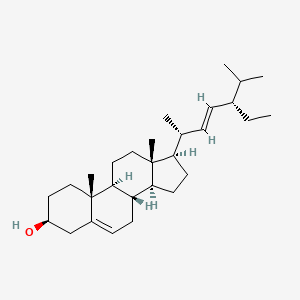

1. Poriferasterol
1. Stigmasterin
2. 83-48-7
3. Beta-stigmasterol
4. Stigmasta-5,22-dien-3-ol, (3b,22e)-
5. Stigmasta-5,22-dien-3beta-ol
6. .beta.-stigmasterol
7. (24s)-5,22-stigmastadien-3beta-ol
8. Stigmasta-5,22-dien-3-beta-ol
9. Stigmasta-5,22-dien-3-ol, (3beta,22e)-
10. (3beta,22e)-stigmasta-5,22-dien-3-ol
11. 99wuk5d0y8
12. Chebi:28824
13. Stigmasta-5,22e-dien-3beta-ol
14. Nsc 8095
15. Nsc-8095
16. .delta.5,22-stigmastadien-3.beta.-ol
17. (3.beta.,22e)-stigmasta-5,22-dien-3-ol
18. Stigmasta-5,22-dien-3-ol
19. Mfcd00003630
20. (3s,8s,9s,10r,13r,14s,17r)-17-[(e,1r,4s)-4-ethyl-1,5-dimethyl-hex-2-enyl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-ol
21. 3beta-hydroxy-24-ethyl-5,22-cholestadiene
22. Unii-99wuk5d0y8
23. Serposterol
24. B-stigmasterol
25. Stigmasta-5,22-dien-3.beta.-ol
26. Ccris 7476
27. D5-stigmasterol
28. Hsdb 7683
29. Delta5-stigmasterol
30. Einecs 201-482-7
31. Stimasterol
32. Stigmasterol, ~95%
33. Stigmasta-5,22-dien-3-ol, (3beta)-
34. Stigmasterol [mi]
35. Stigmasterol [hsdb]
36. Wulzen Anti-stiffness Factor
37. Schembl23999
38. .delta.5-stigmasterol
39. Stigmasterol [who-dd]
40. Stigmasta-5,22t-dien-3b-ol
41. Chembl400247
42. Stigmasta-5,22-dien-3-b-ol
43. Dtxsid801015733
44. Delta5,22-stigmastadien-3beta-ol
45. Hy-n0131
46. Zinc4096712
47. (24s)-5,22-stigmastadien-3b-ol
48. Bdbm50376364
49. Lmst01040123
50. S2361
51. Stl570256
52. 24afh-stigmasta-5,22t-dien-3b-ol
53. Akos022168193
54. Cs-7746
55. (22e)-stigmasta-5,22-dien-3beta-ol
56. (24s)-stigmast-5,22-dien-3beta-ol
57. (24xh)-stigmasta-5,22t-dien-3b-ol
58. 24-ethyl-5,22-cholestadien-3beta-ol
59. 5,22-cholestadien-24-ethyl-3beta-ol
60. Guinea-pig-anti-stiffness Factor
61. (24afh)-stigmasta-5,22t-dien-3b-ol
62. (24x)-ethylcholesta-5,22-dien-3b-ol
63. Ncgc00142599-03
64. (3b,22e)-stigmasta-5,22-dien-3-ol
65. (3s,8s,9s,10r,13r,14s,17r)-17-((2r,5s,e)-5-ethyl-6-methylhept-3-en-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-ol
66. (3s,8s,9s,10r,13r,14s,17r)-17-[(e,2r,5s)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3-ol
67. 14-((2e)(4s,1r)-4-ethyl-1,5-dimethylhex-2-enyl)(1s,5s,10s,11s,2r,14r,15r)-2,15 -dimethyltetracyclo[8.7.0.0<2,7>.0<11,15>]heptadec-7-en-5-ol
68. 24x-24-ethylcholest-5,22-dien-3b-ol
69. As-15473
70. 24-ethyl-5,22-cholestadien-3.beta.-ol
71. 5,22-cholestadien-24beta-ethyl-3beta-ol
72. Rac-(24xh)-stigmasta-5,22t-dien-3b-ol
73. (24s)-24-ethylcholesta-5,22-dien-3beta-ol
74. C05442
75. Q425004
76. Stigmasterol (constituent Of Pygeum) [dsc]
77. Q-201746
78. Stigmasterol (constituent Of Saw Palmetto) [dsc]
79. 3.beta.-hydroxy-24-ethyl-.delta.(sup 5,22)-cholestadiene
80. Stigmasterol, Certified Reference Material, 10 Mg/ml In Chloroform
81. 17-(4-ethyl-1,5-dimethyl-hex-2-enyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-ol
| Molecular Weight | 412.7 g/mol |
|---|---|
| Molecular Formula | C29H48O |
| XLogP3 | 8.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Exact Mass | 412.370516150 g/mol |
| Monoisotopic Mass | 412.370516150 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 674 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL/ ... The primary aim of this study was to determine the efficacy of a low-fat spread enriched with plant sterols in reducing total and low density lipoprotein-cholesterol (LDL-C) concentrations in primary hypercholesterolemia. The secondary objective was to evaluate whether patients receiving a lipid-lowering drug (fibrate) might differ in their response to plant sterols. The study was a randomized, double-blind, placebo-controlled two-period cross-over trial with two treatments and three periods. Both treatment periods lasted 2 months, with a washout period (2 months) between them. Spread enriched with plant sterols was compared to non-enriched control spread. Fortified fat spread provided 1.6 g/day of plant sterols derived from edible vegetable oils and fatty acids from sunflower seed oil. The plant sterol content consisted of sitosterol esters (50%), campesterol esters (25%), stigmasterol esters (20%) and 10% of other esters. Data in 53 hypercholesterolemic patients (31 females and 22 males) who completed the study were as follows: patients were 58+/-12 years of age with mean body mass index 23.5+/-2.8 kg/m2 (mean+/-SD). No adverse side-effects of the diet were reported. Plasma total cholesterol and LDL-C concentrations were significantly reduced by 6.4% and 8.8%, respectively, after using the spread enriched in plant sterols, as compared to controls (0.0% and 1.3%, respectively). No effect on high density lipoprotein-cholesterol (HDL-C) and lipoprotein(a) concentrations was detected. When subjects were divided in two subgroups according to fibrate treatment, supplementation with phytosterols decreased plasma cholesterol and LDL-C by 8.5% and 11.1%, respectively in the subgroup of patients treated with fibrates... /It was concluded/ that phytosterol-enriched spread is a useful adjunctive therapy for hypercholesterolemic patients.
PMID:11522112 Nigon F et al; Clin Chem Lab Med 39 (7): 634-40 (2001)
/EXPL/ The commonly consumed plant sterols are sitosterol, stigmasterol and campesterol which are predominantly supplied by vegetable oils. ... The nutritional interest derives from the fact that the sterols have a similar structure to cholesterol, and have the capacity to lower plasma cholesterol and LDL cholesterol. Since the morbidity and mortality from cardiovascular disease have been dramatically reduced using cholesterol-lowering drugs (statins), the interest in plant sterols lies in their potential to act as a natural preventive dietary product.
Piironen V et al; J Sci Food Agric 80 (7): 939-66 (2000)
/EXPL/ A study was conducted in 12 healthy males and 12 healthy females (mean age 36 years, mean body mass index 24 kg/m2), to determine the effect of a margarine enriched with phytosterol esters on fecal short-chain fatty acids (SCFAs) and fecal bacterial enzyme activities, viable fecal microflora count, female sex hormones and serum cholesterol concentrations. The study design was a two-period, parallel dosing, randomized, placebo-controlled dietary study. Under controlled dietary conditions, participants consumed 40 g of the control margarine for 21 and 28 consecutive days for males and females, respectively. This was followed immediately by the second part of the study where subjects were equally and randomly allocated to consume daily 40 g of either the control or the test margarine, containing 8.6 g vegetable oil phytosterols (a mixture of beta-sitosterol, campesterol and stigmasterol), also for 21 or 28 days. All females were shown to have a regular menstrual cycle and were on an established method of contraception not involving oral contraceptives. When compared with the control group values, the test group showed a significant reduction in serum total and LDL cholesterol concentrations of 18 and 23% (P < 0.001; P < 0.001) respectively, in fecal lactic acid concentration (P = 0.039) and in serum progesterone levels (P = 0.021). There were no other significant treatment effects. Within each group a number of significant changes occurred compared to baseline. In the test group, fecal lactic acid concentration and the ratio of acetic acid:total SCFA; and the ratio of butyric acid:total SCFA, in the control group were both significantly reduced (P = 0.016). Compared to baseline, azo-reductase activity was significantly reduced in the control group (P = 0.047). Total fecal aerobes (P = 0.028), lactobacilli (P = 0.003) and staphylococci (P = 0.025) content was also significantly reduced in the control group, while in the test group only lactobacilli content was reduced (P = 0.019). Of the significant findings reported in this study, none was considered to be of biological importance except the beneficial reduction in serum total and LDL-cholesterol concentrations...
PMID:10654588 Ayesh R et al; Food Chem Toxicol 37 (12): 1127-38 (1999)
... Metabolism of plant sterols and squalene administered intravenously in the form of lipid emulsion mimicking chylomicrons (CM) /was studied/. The CM-like lipid emulsion was prepared by dissolving squalene in commercially available Intralipid. The emulsion was given as an intravenous bolus injection of 30 mL containing 6.3 mg of cholesterol, 1.9 mg of campesterol, 5.7 mg of sitosterol, 1.6 mg of stigmasterol, 18.1 mg of squalene, and 6 g of triglycerides in six healthy volunteers. Blood samples were drawn from the opposite arm before and serially 2.5 -180 min after the injections. The decay of CM squalene, plant sterols, and triglycerides was monoexponential. The half-life of CM squalene was 74 +/- 8 min, that of campesterol was 37 +/- 5 min (P < 0.01 from squalene), and those of sitosterol, stigmasterol, and triglycerides were 17 +/- 2, 15 +/- 1, and 17 +/- 2 min, respectively (P < 0.01 from squalene and campesterol). The CM squalene concentration still exceeded the baseline level 180 min after injection (P = 0.02), whereas plant sterols and triglycerides returned to the baseline level between 45 and 120 min after injection. The half-lives of squalene and campesterol were positively correlated with their fasting CM concentrations. In addition, VLDL squalene, campesterol, and triglyceride concentrations, VLDL, LDL, and HDL sitosterol concentrations, as well as VLDL and LDL stigmasterol concentrations were increased significantly...
PMID:11369807 Relas H et al; J Lipid Res 42 (6): 988-94 (2001)
Rats were dosed by oral gavage with 14C-labelled samples of cholesterol, beta-sitosterol or beta-sitostanol or (3)H-labelled samples of beta-sitostanol, campesterol, campestanol or stigmasterol dissolved in sunflower seed oil. Urine and feces were collected for up to 96 hours after dosing. ... Animals were sacrificed and either prepared for whole body autoradiography or tissues and carcass remains were assayed for 14C or (3)H. The overall absorption of phytosterols was low as judged by tissue and carcass levels of radioactivity. Elimination from the body was mainly in the feces and was initially very rapid, but traces of material were still being excreted at 4 days after dosing. While total absorption of the phytosterols could not be fully quantified without biliary excretion data, it was clear that cholesterol was absorbed to the greatest extent (27% of the dose in females at 24 hours). Campesterol (13%) was absorbed more than beta-sitosterol and stigmasterol (both 4%) which were absorbed more than beta-sitostanol and campestanol (1-2%). The absorption of phytosterols was slightly greater in females than males. For each test material, the overall pattern of tissue distribution of radioactivity was similar, with the adrenal glands, ovaries and intestinal epithelia showing the highest levels and the longest retention of radioactivity.
PMID:10828500 Sanders DJ et al; Food Chem Toxicol 38 (6): 485-91 (2000)
Intestinal absorption of cholesterol, campesterol, campestanol, stigmasterol and sitosterol were measured in 10 healthy subjects by an intestinal perfusion technique over a 50 cm segment of the upper jejunum using sitostanol as non-absorbable marker. Cholesterol absorption was highest and averaged 33%., whereas the absorption rate of sitosterol averaged 4.2% and of stigmasterol 4.8%....
PMID:8143759 Heinemann T et al; Eur J CLin Invest 23 (12): 827-31 (1993)
To study the effects of dietary stigmasterol on sterol and bile acids metabolism, Wistar rats were fed diets containing various amounts of stigmasterol. Feeding high stigmasterol doses (11, 26 or 52 mg/day) led to increased cholesterol, coprostanol and bile acid output. These effects were dose-dependent, and likely to be related to the inhibitory effect of plant sterols on cholesterol absorption. Moreover, it accounts for the beneficial effect of the stigmasterol on cholesterol lowering.
PMID:2516429 Andriamiarina R et al; Ann Nutr Metab 33 (5): 297-303 (1989)
Tobacco sterols (cholesterol, beta-sitosterol, campesterol, and stigmasterol) are present in tobacco smoke and appear in plasma of mammals exposed to cigarette smoke. Because tobacco sterols may be important in the pathogenesis of smoking-induced lung and vascular diseases, ... the pattern of deposition of cigarette sterols in the lungs and appearance of cigarette sterols in plasma and body organs of rats /were studied/. After exposure to twenty 5 mL "puffs" of smoke from tobacco labeled with [4-14C]cholesterol or beta-[4-14C]sitosterol, rats were killed just after exposure (day 0) and on days 2, 5, 8, 11, 15, and 30, and the lungs and selected body organs analyzed for activity. ... Cigarette sterols /were/ associated with particulates in cigarette smoke, deposited mostly in distal airspaces and parenchyma of the lungs, and appear in plasma and several body organs for more than 30 days after this single exposure to cigarette smoke. Bronchoalveolar lavage fluid contained relatively small amounts of radiolabel for only the first few days, suggesting that most of the sterols were rapidly incorporated in lung parenchyma...
Holden WE et al; J Lab Clin Med 112 (2)L 216-22 (1988)
Metabolism of phytosterols was investigated using rat feces and liver microsomes. Feces were collected after phytosterols (a well characterized mixture of beta-sitosterol 40%, campesterol 30% and dihydrobrasicasterol) were administered orally (0.5 g/kg) to rats. Metabolites of phytosterols were identified using GC/MS. Three peaks were eluted at 12.47, 12.65, 12.87 min and had characteristic molecular ions m/z 428, 430, 432, respectively. Three fecal metabolites were identified as androstadienedione, androstenedione, and androstanedione. No metabolites could be detected in the rat liver microsomal reaction mixture. The results suggest that the metabolites of phytosterols in rat feces are formed by oxidation at 3- position, saturation at 5- and 6- position, and 17- side chain cleavage in the rat large intestine.
PMID:11156182 Song YS et al; Arch Pharm Res 23 (6): 599-604 (2000)
Plant sterols are an essential component of the membranes of all eukaryotic organisms. They are either synthesized de novo or taken up from the environment. Their function appears to be to control membrane fluidity and permeability, although some plant sterols have a specific function in signal transduction. The phytosterols are products of the isoprenoid pathway. The dedicated pathway to sterol synthesis in photosynthetic plants occurs at the squalene stage through the activity of squalene synthetase. Although the activity of 3-hydroxymethyl-3-glutaryl coenzyme A (HGMR) is rate-limiting in the synthesis of cholesterol, this does not appear to be the case with the plant sterols. Up-regulation of HGMR appears to increase the biosynthesis of cycloartenol but not the delta5-sterols. A decline in sterol synthesis is associated with a suppression of squalene synthetase activity, which is probably a critical point in controlling carbon flow and end-product formation. The major post-squalene biosynthetic pathway is regulated by critical rate-limiting steps such as the methylation of cycloartenol into cycloeucalenol. Little is known about the factors controlling the biosynthesis of the end-point sterol esters or stanols.
Piironen V et al; J Sci Food Agric 80 (7): 939-66 (2000)
Market Place

ABOUT THIS PAGE
10
PharmaCompass offers a list of Stigmasterol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Stigmasterol manufacturer or Stigmasterol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Stigmasterol manufacturer or Stigmasterol supplier.
PharmaCompass also assists you with knowing the Stigmasterol API Price utilized in the formulation of products. Stigmasterol API Price is not always fixed or binding as the Stigmasterol Price is obtained through a variety of data sources. The Stigmasterol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Stigmasterol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Stigmasterol, including repackagers and relabelers. The FDA regulates Stigmasterol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Stigmasterol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Stigmasterol supplier is an individual or a company that provides Stigmasterol active pharmaceutical ingredient (API) or Stigmasterol finished formulations upon request. The Stigmasterol suppliers may include Stigmasterol API manufacturers, exporters, distributors and traders.
Stigmasterol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Stigmasterol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Stigmasterol GMP manufacturer or Stigmasterol GMP API supplier for your needs.
A Stigmasterol CoA (Certificate of Analysis) is a formal document that attests to Stigmasterol's compliance with Stigmasterol specifications and serves as a tool for batch-level quality control.
Stigmasterol CoA mostly includes findings from lab analyses of a specific batch. For each Stigmasterol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Stigmasterol may be tested according to a variety of international standards, such as European Pharmacopoeia (Stigmasterol EP), Stigmasterol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Stigmasterol USP).

