
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
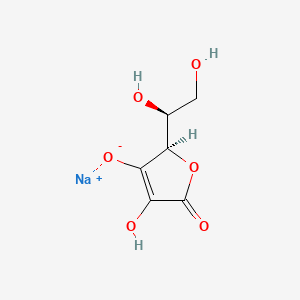

1. Acid, Ascorbic
2. Acid, L-ascorbic
3. Ascorbate, Ferrous
4. Ascorbate, Magnesium
5. Ascorbate, Sodium
6. Ascorbic Acid
7. Ascorbic Acid, Monosodium Salt
8. Di-l-ascorbate, Magnesium
9. Ferrous Ascorbate
10. Hybrin
11. L Ascorbic Acid
12. L-ascorbic Acid
13. Magnesium Ascorbate
14. Magnesium Ascorbicum
15. Magnesium Di L Ascorbate
16. Magnesium Di-l-ascorbate
17. Magnorbin
18. Vitamin C
1. 134-03-2
2. Sodium L-ascorbate
3. L-ascorbic Acid Sodium Salt
4. Vitamin C Sodium
5. Ascorbic Acid Sodium Salt
6. Monosodium L-ascorbate
7. Vitamin C Sodium Salt
8. Ascorbicin
9. Sodascorbate
10. (+)-sodium L-ascorbate
11. L-ascorbic Acid, Monosodium Salt
12. Natrii Ascorbas
13. Vitamin C, Sodium Salt
14. Ascorbate
15. Ascorbate De Sodium
16. Ascorbin
17. Cevalin
18. L-ascorbic Acid Sodium
19. Ins No.301
20. L-ascorbic Acid (sodium Salt)
21. Ins-301
22. L-ascorbate, Sodium
23. Sodium (r)-2-((s)-1,2-dihydroxyethyl)-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
24. S033eh8359
25. Cebitate
26. Aminofenitrooxon
27. E-301
28. Sodium (2r)-2-[(1s)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2h-furan-3-olate
29. Iskia-c
30. Natri-c
31. Sodium (l)-ascorbate
32. Sodiumascorbate
33. Ascorbato Sodico
34. Ascorbato Sodico [dcit]
35. Sodium;(2r)-2-[(1s)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2h-furan-3-olate
36. Mfcd00082340
37. Natrii Ascorbas [inn-latin]
38. Ascorbic Acid Sodium Derivative
39. Ccris 3291
40. Hsdb 694
41. 3-oxo-l-gulofuranolactone Sodium
42. Hbl 508
43. Ascorbate De Sodium [inn-french]
44. Einecs 205-126-1
45. Tianafacacid
46. Sodium Ascorbate [usp:inn]
47. Unii-s033eh8359
48. Sodium Derivative Of 3-oxo-l-gulofuranolactone
49. Ascorbic Acid Sodium
50. E301
51. Sodium L-ascorbate Salt
52. Dsstox_cid_105
53. Ec 205-126-1
54. Schembl3745
55. Dsstox_rid_75369
56. Dsstox_gsid_20105
57. Sodium Ascorbate [ii]
58. L(+)ascorbic Acid Sodium Salt
59. Sodium Ascorbate [fcc]
60. Sodium Ascorbate [inn]
61. Chembl591665
62. Sodium Ascorbate [hsdb]
63. Sodium Ascorbate [inci]
64. Dtxsid0020105
65. Sodium Ascorbate [vandf]
66. Hy-b0166a
67. Sodium Ascorbate [mart.]
68. Chebi:113451
69. Sodium Ascorbate [usp-rs]
70. Sodium Ascorbate [who-dd]
71. L-ascorbic Acid Sodium Salt,(s)
72. L-ascorbic Acid - Monosodium Salt
73. Tox21_300556
74. Akos015895058
75. L-ascorbic Acid, Sodium Salt (1:1)
76. Sodium Ascorbate [orange Book]
77. Ascorbic Acid Sodium Salt [mi]
78. Cs-6063
79. Db14482
80. Sodium Ascorbate [ep Monograph]
81. Sodium (2r)-2-[(1s)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
82. Sodium Ascorbate [usp Monograph]
83. Ncgc00254355-01
84. Bp-30077
85. Cas-134-03-2
86. A0539
87. B1834
88. E80761
89. A806721
90. Q424551
91. J-006471
92. Sodium (2r)-2-[(1s)-1,2-bis(oxidanyl)ethyl]-4-oxidanyl-5-oxidanylidene-2h-furan-3-olate
93. Sodium(r)-2-((s)-1,2-dihydroxyethyl)-4-hydroxy-5-oxo-2,5-dihydrofuran-3-olate
| Molecular Weight | 198.11 g/mol |
|---|---|
| Molecular Formula | C6H7NaO6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 198.01403222 g/mol |
| Monoisotopic Mass | 198.01403222 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 237 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antioxidants; Free Radical Scavengers
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ascorbic acid and calcium and sodium ascorbates are used as antoxidants in pharmaceutical manufacturing and in the food industry.
Sweetman SC (ed), Martindale: The Complete Drug Reference. London: Pharmaceutical Press (2009), p.1985.
In 20 patients in acute asthmatic crisis, 16 recovered promptly after receiving 6 g sodium ascorbate iv. Chronic oral treatment (0.6-1 g/day/60 days) with Na ascorbate prevented asthmatic symptoms in 18/25 asthmatic patients.
Miyares C et al; Rev Cubana Med 10 (5): 469 (1971)
8 patients with hyphema were treated with iv glycerin in combination with sodium ascorbate. The results showed that glycerol in combination with sodium ascorbate diminished the hemorrhage in eye within 12-24 hr.
PMID:7272018 Latinovic S et al; Boll Chim Farm 120: 156-8 (1981)
For more Therapeutic Uses (Complete) data for Sodium ascorbate (6 total), please visit the HSDB record page.
Each gram of sodium ascorbate contains approximately 5 mEq of sodium; this should be considered when the drug is used in patients on salt-restricted diets.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
Ascorbic acid, the reduced form of vitamin C, functions as a potent antioxidant as well as in cell differentiation. Ascorbate is taken up by mammalian cells through the specific sodium/ascorbate co-transporters SVCT1 and SVCT2. Although skeletal muscle contains about 50% of the whole-body vitamin C, the expression of SVCT transporters has not been clearly addressed in this tissue. ... This work ... analyzed the expression pattern of SVCT2 during embryonic myogenesis using the chick as model system. ... Immunohistochemical analyses showed that SVCT2 is preferentially expressed by type I slow-twitch muscle fibers throughout chick myogenesis as well as in post-natal skeletal muscles of several species, including human...
Low M et al; Histochem Cell Biol 131 (5): 565-74 (2009). Available from, as of March 15, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=19125272
Humans use two sodium-ascorbate cotransporters (hSVCT1 and hSVCT2) for transporting the dietary essential micronutrient ascorbic acid, the reduced and active form of vitamin C. Although the human liver plays a pivotal role in regulating and maintaining vitamin C homeostasis, vitamin C transport physiology and regulation of the hSVCT systems in this organ have not been well defined. Thus, this research used a human hepatic cell line (HepG2), confirming certain results with primary human hepatocytes and determined the initial rate of ascorbic acid uptake to be Na(+) gradient, pH dependent, and saturable as a function of concentration over low and high micromolar ranges. Additionally, hSVCT2 protein and mRNA are expressed at higher levels in HepG2 cells and native human liver, and the cloned hSVCT2 promoter has more activity in HepG2 cells. Results using short interfering RNA suggest that in HepG2 cells, decreasing hSVCT2 message levels reduces the overall ascorbic acid uptake process more than decreasing hSVCT1 message levels. Activation of PKC intracellular regulatory pathways caused a downregulation in ascorbic acid uptake not mediated by a single predicted PKC-specific amino acid phosphorylation site in hSVCT1 or hSVCT2. However, PKC activation causes internalization of hSVCT1 but not hSVCT2. Examination of other intracellular regulatory pathways on ascorbic acid uptake determined that regulation also potentially occurs by PKA, PTK, and Ca(2+)/calmodulin, but not by nitric oxide-dependent pathways...
Reidling JC et al; Am J Physiol. Gastrointest Liver Physiol 295 (6): 1217-27 (2008). Available from, as of March 15, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=18845575
... Adrenal cortex is closely associated with ascorbate metabolism ... Hydrocortisone was reported ... to stimulate synthesis of ascorbate from gluconolactone, but deoxycorticosterone or aldosterone caused ... increase in ascorbate excretion in normal or adrenalectomized rats...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 607
Mechanism of action of ascorbate is a superoxide radical scavenger.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 524
... Sodium ascorbate decreases cellular iron uptake by melanoma cells in a dose- and time-dependent fashion, indicating that intracellular iron levels may be a critical factor in sodium ascorbate-induced apoptosis. Indeed, sodium ascorbate-induced apoptosis is enhanced by the iron chelator, desferrioxamine (DFO) while it is inhibited by the iron donor, ferric ammonium citrate (FAC). Moreover, the inhibitory effects of sodium ascorbate on intracellular iron levels are blocked by addition of transferrin, suggesting that transferrin receptor (TfR) dependent pathway of iron uptake may be regulated by sodium ascorbate. Cells exposed to sodium ascorbate demonstrated down-regulation of TfR expression and this precedes sodium ascorbate-induced apoptosis. Taken together, sodium ascorbate-mediated apoptosis appears to be initiated by a reduction of TfR expression, resulting in a down-regulation of iron uptake followed by an induction of apoptosis...
Kang JS et al; J Cell Physiol 204 (1): 192-7 (2005). Available from, as of March 15, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15672419
Humans use two sodium-ascorbate cotransporters (hSVCT1 and hSVCT2) for transporting the dietary essential micronutrient ascorbic acid, the reduced and active form of vitamin C. Although the human liver plays a pivotal role in regulating and maintaining vitamin C homeostasis, vitamin C transport physiology and regulation of the hSVCT systems in this organ have not been well defined. Thus, this research used a human hepatic cell line (HepG2), confirming certain results with primary human hepatocytes and determined the initial rate of ascorbic acid uptake to be Na(+) gradient, pH dependent, and saturable as a function of concentration over low and high micromolar ranges. Additionally, hSVCT2 protein and mRNA are expressed at higher levels in HepG2 cells and native human liver, and the cloned hSVCT2 promoter has more activity in HepG2 cells. Results using short interfering RNA suggest that in HepG2 cells, decreasing hSVCT2 message levels reduces the overall ascorbic acid uptake process more than decreasing hSVCT1 message levels. Activation of PKC intracellular regulatory pathways caused a downregulation in ascorbic acid uptake not mediated by a single predicted PKC-specific amino acid phosphorylation site in hSVCT1 or hSVCT2. However, PKC activation causes internalization of hSVCT1 but not hSVCT2. Examination of other intracellular regulatory pathways on ascorbic acid uptake determined that regulation also potentially occurs by PKA, PTK, and Ca(2+)/calmodulin, but not by nitric oxide-dependent pathways...
Reidling JC et al; Am J Physiol. Gastrointest Liver Physiol 295 (6): 1217-27 (2008). Available from, as of March 15, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=18845575
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4743
Submission : 1982-11-18
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-05-29
Pay. Date : 2018-03-22
DMF Number : 23686
Submission : 2010-04-03
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-10-19
Pay. Date : 2017-01-10
DMF Number : 31184
Submission : 2016-12-21
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38718
Submission : 2023-12-15
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2012-262 - Rev 03
Status : Valid
Issue Date : 2024-02-22
Type : Chemical
Substance Number : 1791

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

Certificate Number : CEP 2002-254 - Rev 05
Status : Valid
Issue Date : 2025-07-17
Type : Chemical
Substance Number : 1791

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] NDC Package Code : 72783-1002
Start Marketing Date : 2020-04-22
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 63238-3050
Start Marketing Date : 2017-12-20
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 63238-3051
Start Marketing Date : 2024-02-13
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 49452-6581
Start Marketing Date : 2022-01-03
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Asence Pharma private Limited is a subsidiary company of Asence INC. USA - a New York based pharmaceutical company. Asence specializes in the supply of quality pharmaceutical prepa...

About the Company : Bajaj Healthcare Ltd. is a company established in 1993, services various Pharmaceuticals, Nutraceutical and Food industries globally with a spirit of scrupulousness. Bajaj Healt...

About the Company : CSPC Pharmaceutical Group Limited ("CSPC") (Stock code: 1093. HK) is a leading pharmaceutical group in China. It was established in 1997. Since the very beginning, CSPC has taken �...

About the Company : India Phosphate, established in 2007, is a leading processor, exporter, and supplier of high-quality Calcium Phosphate. Known for its purity, accuracy, and cost-effectiveness, our ...

About the Company : Jiaan Biotech is counted as a reliable Manufacturer and Supplier of quality oriented Mineral Supplements. The Manufacturing Unit is based in Pithampur Industrial Area , Madhya Prad...

About the Company : Reckon Organics Private Ltd, formerly RecckonPharmachem Pvt. Ltd., specializes in manufacturing Ascorbic Acid and its derivatives. Established in 2008, the company supplies bulk dr...

About the Company : Royal DSM, founded in 1902, is a global, purpose-led health, nutrition, and bioscience company that specializes in human and animal health and nutrition solutions. Its primary goal...

About the Company : Shreenath Chemicals stands as a prominent producer of bulk drugs, drug intermediates, and fine chemicals. Operating from two advanced manufacturing facilities located in M.I.D.C. T...

About the Company : Zhejiang NHU Co., Ltd. was founded in 1999 and listed in 2004 (stock code 002001). Since its establishment, the company has always adhered to the concept of innovation driven devel...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
62
PharmaCompass offers a list of Sodium Ascorbate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Ascorbate manufacturer or Sodium Ascorbate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Ascorbate manufacturer or Sodium Ascorbate supplier.
PharmaCompass also assists you with knowing the Sodium Ascorbate API Price utilized in the formulation of products. Sodium Ascorbate API Price is not always fixed or binding as the Sodium Ascorbate Price is obtained through a variety of data sources. The Sodium Ascorbate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A (+)-Sodium L-ascorbate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of (+)-Sodium L-ascorbate, including repackagers and relabelers. The FDA regulates (+)-Sodium L-ascorbate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. (+)-Sodium L-ascorbate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of (+)-Sodium L-ascorbate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A (+)-Sodium L-ascorbate supplier is an individual or a company that provides (+)-Sodium L-ascorbate active pharmaceutical ingredient (API) or (+)-Sodium L-ascorbate finished formulations upon request. The (+)-Sodium L-ascorbate suppliers may include (+)-Sodium L-ascorbate API manufacturers, exporters, distributors and traders.
click here to find a list of (+)-Sodium L-ascorbate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A (+)-Sodium L-ascorbate DMF (Drug Master File) is a document detailing the whole manufacturing process of (+)-Sodium L-ascorbate active pharmaceutical ingredient (API) in detail. Different forms of (+)-Sodium L-ascorbate DMFs exist exist since differing nations have different regulations, such as (+)-Sodium L-ascorbate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A (+)-Sodium L-ascorbate DMF submitted to regulatory agencies in the US is known as a USDMF. (+)-Sodium L-ascorbate USDMF includes data on (+)-Sodium L-ascorbate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The (+)-Sodium L-ascorbate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of (+)-Sodium L-ascorbate suppliers with USDMF on PharmaCompass.
A (+)-Sodium L-ascorbate CEP of the European Pharmacopoeia monograph is often referred to as a (+)-Sodium L-ascorbate Certificate of Suitability (COS). The purpose of a (+)-Sodium L-ascorbate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of (+)-Sodium L-ascorbate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of (+)-Sodium L-ascorbate to their clients by showing that a (+)-Sodium L-ascorbate CEP has been issued for it. The manufacturer submits a (+)-Sodium L-ascorbate CEP (COS) as part of the market authorization procedure, and it takes on the role of a (+)-Sodium L-ascorbate CEP holder for the record. Additionally, the data presented in the (+)-Sodium L-ascorbate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the (+)-Sodium L-ascorbate DMF.
A (+)-Sodium L-ascorbate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. (+)-Sodium L-ascorbate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of (+)-Sodium L-ascorbate suppliers with CEP (COS) on PharmaCompass.
A (+)-Sodium L-ascorbate written confirmation ((+)-Sodium L-ascorbate WC) is an official document issued by a regulatory agency to a (+)-Sodium L-ascorbate manufacturer, verifying that the manufacturing facility of a (+)-Sodium L-ascorbate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting (+)-Sodium L-ascorbate APIs or (+)-Sodium L-ascorbate finished pharmaceutical products to another nation, regulatory agencies frequently require a (+)-Sodium L-ascorbate WC (written confirmation) as part of the regulatory process.
click here to find a list of (+)-Sodium L-ascorbate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing (+)-Sodium L-ascorbate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for (+)-Sodium L-ascorbate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture (+)-Sodium L-ascorbate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain (+)-Sodium L-ascorbate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a (+)-Sodium L-ascorbate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of (+)-Sodium L-ascorbate suppliers with NDC on PharmaCompass.
(+)-Sodium L-ascorbate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of (+)-Sodium L-ascorbate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right (+)-Sodium L-ascorbate GMP manufacturer or (+)-Sodium L-ascorbate GMP API supplier for your needs.
A (+)-Sodium L-ascorbate CoA (Certificate of Analysis) is a formal document that attests to (+)-Sodium L-ascorbate's compliance with (+)-Sodium L-ascorbate specifications and serves as a tool for batch-level quality control.
(+)-Sodium L-ascorbate CoA mostly includes findings from lab analyses of a specific batch. For each (+)-Sodium L-ascorbate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
(+)-Sodium L-ascorbate may be tested according to a variety of international standards, such as European Pharmacopoeia ((+)-Sodium L-ascorbate EP), (+)-Sodium L-ascorbate JP (Japanese Pharmacopeia) and the US Pharmacopoeia ((+)-Sodium L-ascorbate USP).
