
Synopsis
Synopsis
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
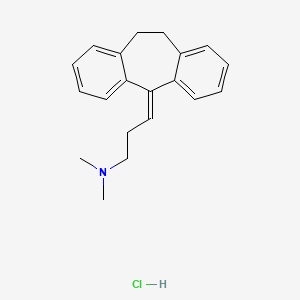

1. Amineurin
2. Amitrip
3. Amitriptylin Beta
4. Amitriptylin Desitin
5. Amitriptylin Neuraxpharm
6. Amitriptylin Rph
7. Amitriptylin-neuraxpharm
8. Amitriptyline
9. Amitrol
10. Anapsique
11. Apo Amitriptyline
12. Apo-amitriptyline
13. Damilen
14. Desitin, Amitriptylin
15. Domical
16. Elavil
17. Endep
18. Laroxyl
19. Lentizol
20. Novoprotect
21. Rph, Amitriptylin
22. Saroten
23. Sarotex
24. Syneudon
25. Triptafen
26. Tryptanol
27. Tryptine
28. Tryptizol
1. 549-18-8
2. Amitriptyline Hcl
3. Annoyltin
4. Tryptizol
5. Domical
6. Syneudon
7. Endep
8. Lentizol
9. Novoprotect
10. Etravil
11. Saroten
12. Elavil Hydrochloride
13. Amineurin
14. Amitril
15. Amitrip
16. Anapsique
17. Tryptine
18. Amavil
19. Amitid
20. Ami-anelun
21. Apo-amitriptyline
22. Daprimen
23. Elatrolet
24. Maxivalet
25. Miketorin
26. Mitaptyline
27. Rantoron
28. Sarotena
29. Trepiline
30. Triptizol
31. Trytomer
32. Vanatrip
33. Yamanouchi
34. Amilit
35. Amiplin
36. Amiprin
37. Amyline
38. Amyzol
39. Belpax
40. Elatrol
41. Enafon
42. Etrafon
43. Kyliran
44. Larozyl
45. Levate
46. Pinsanu
47. Pinsaun
48. Teperin
49. Triavil
50. Tridep
51. Tripta
52. Trynol
53. Damilen Hydrochloride
54. Amitriptyline Chloride
55. Saroten Retard
56. Tryptizol Retard
57. Limbitrol Ds
58. Tryptacap Hydrochloride
59. Adt-zimaia
60. Oasil-m
61. Redomex
62. Proheptadien Monohydrochloride
63. Amitriptyline (hydrochloride)
64. Sk-amitriptyline Chloride
65. Limbitrol
66. Sarotex
67. Nih 10794
68. Ccris 7092
69. Amitryptiline Hydrochloride
70. Nsc-104210
71. 26lud4jo9k
72. Annolytin
73. Mls000028437
74. Component Of Etrafon
75. Component Of Triavil
76. 5-(3-(dimethylamino)propylidene) Dibenzosuberane Hcl
77. 3-(10,11-dihydro-5h-dibenzo[a,d][7]annulen-5-ylidene)-n,n-dimethylpropan-1-amine Hydrochloride
78. 1-propanamine, 3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-ylidene)-n,n-dimethyl-, Hydrochloride
79. Novotriptyn
80. Nornaln
81. Smr000058368
82. 549-18-8 (hcl)
83. Amitryptyline Hydrochloride
84. 5-(3-dimethylaminopropylidene)dibenzo(a,d)(1,4)cycloheptadiene Hydrochloride
85. Dsstox_cid_13187
86. Dsstox_rid_79055
87. Dsstox_gsid_33187
88. Amitriptyline Hydrochloride 100 Microg/ml In Acetonitrile
89. 1-propanamine, 3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-, Hydrochloride
90. 3-(10,11-dihydro-5h-dibenzo[a,d][7]annulen-5-ylidene)-n,n-dimethyl-1-propanamine Hydrochloride
91. 3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-1-propanamine Hydrochloride
92. Nsc169910
93. Wln: L C676 By&t&j Bu3n1&1 &gh
94. Amitriptyline Hydrochloride [jan]
95. Sr-01000003046
96. Ncgc00015095-09
97. Cas-549-18-8
98. Einecs 208-964-6
99. Mfcd00012537
100. Amitryptylline Hydrochloride
101. Unii-26lud4jo9k
102. Nsc 104210
103. Amilent
104. Sylvemid
105. Amicen
106. Euplit
107. Uxen
108. 5-(3-dimethylaminopropylidene)dibenzo[a,4]cycloheptadiene Hydrochloride
109. 5-[3-(dimethylamino)propylidene]dibenzo[a,4]cycloheptadiene Hydrochloride
110. 1-propanamine,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-, Hydrochloride
111. 5-(3-dimethylaminopropylidene)dibenzo[a,d][1,4]cycloheptadiene Hydrochloride
112. 5h-dibenzo[a,.gamma.-propylamine, 10,11-dihydro-n,n-dimethyl-, Hydrochloride
113. 5h-dibenzo[a,.gamma.-propylamine, 10,11-dihydro-n,n-dimethyl-,hydrochloride
114. Prestwick_20
115. 3-(5,6-dihydrodibenzo[2,1-b:1',2'-e][7]annulen-11-ylidene)-n,n-dimethylpropan-1-amine;hydrochloride
116. Drg-0169
117. Elavil (tn)
118. Amitriptyline Hydrochloride [usp:jan]
119. Cpd000058368
120. Opera_id_618
121. Nortriptyline Impurity F
122. Amitriptylini Hydrochloridum
123. C20h23n.hcl
124. Schembl41079
125. 10,11-dihydro-n,n-dimethyl-5h-dibenzo(a,d)cycloheptene-delta(sup 5,gamma)-propylamine Hydrochloride
126. Mls001055393
127. Mls001074176
128. Mls002222152
129. Spectrum1500117
130. Regid_for_cid_11065
131. Chebi:2667
132. Chembl1200964
133. Dtxsid9033187
134. Hy-b0527a
135. Amitriptyline Hydrochloride ,(s)
136. Amitriptyline Hydrochloride, 99%
137. Hms1568o09
138. Hms1920c13
139. Pharmakon1600-01500117
140. Bcp31573
141. Amitriptyline-[13c3] Hydrochloride
142. Tox21_113122
143. Tox21_201278
144. Tox21_303612
145. Tox21_500112
146. Ccg-38919
147. Nsc104210
148. Nsc755869
149. S3183
150. Akos015994716
151. Tox21_113122_1
152. Ks-1291
153. Lp00112
154. Nc00497
155. Nsc-169910
156. Nsc-755869
157. Amitriptyline Hydrochloride (jp17/usp)
158. Amitriptyline Hydrochloride [mi]
159. Ncgc00015095-15
160. Ncgc00024433-03
161. Ncgc00024433-05
162. Ncgc00093609-01
163. Ncgc00093609-02
164. Ncgc00093609-03
165. Ncgc00093609-04
166. Ncgc00093609-05
167. Ncgc00257393-01
168. Ncgc00258830-01
169. Ncgc00260797-01
170. 5h-dibenzo(a,d)cycloheptene-delta(sup 5),gamma-propylamine, 10,11-dihydro-n,n-dimethyl-, Hydrochloride
171. 5h-dibenzo(a,d)cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-n,n-dimethyl-, Hydrochloride
172. Amitriptyline Hydrochloride [hsdb]
173. Ba164159
174. N,n-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,11,13-hexaenylidene)propan-1-amine;hydrochloride
175. Amitriptyline Hcl;nortriptyline Impurity F
176. Amitriptyline Hydrochloride [mart.]
177. Amitriptyline Hydrochloride [vandf]
178. Amitriptyline Hydrochloride [usp-rs]
179. Amitriptyline Hydrochloride [who-dd]
180. Amitriptyline Hydrochloride [who-ip]
181. A0908
182. Eu-0100112
183. Ft-0662108
184. Ft-0662109
185. Sw196337-3
186. A 8404
187. A16404
188. D00809
189. D81789
190. Amitriptyline Hydrochloride [ep Impurity]
191. A854879
192. Amitriptyline Hydrochloride [ep Monograph]
193. Amitriptyline Hydrochloride [usp Impurity]
194. Amitriptyline Hydrochloride [usp Monograph]
195. Amitriptylini Hydrochloridum [who-ip Latin]
196. Amitriptyline Hydrochloride, >=98% (tlc), Powder
197. Sr-01000003046-2
198. Sr-01000003046-6
199. W-105596
200. Etrafon-a Component Amitriptyline Hydrochloride
201. Limbitrol Component Amitriptyline Hydrochloride
202. Q27254112
203. Amitriptyline Hydrochloride Component Of Etrafon-a
204. Amitriptyline Hydrochloride Component Of Limbitrol
205. F0001-1443
206. Limbitrol Ds Component Amitriptyline Hydrochloride
207. Amitriptyline Hydrochloride Component Of Limbitrol Ds
208. 5-[3-(dimethylamino)propylidene]dibenzosuberane Hydrochloride
209. Amitriptyline Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
210. Amitriptyline Hydrochloride, Drug Standard, 1.0 Mg/ml In Methanol
211. Amitriptyline Hydrochloride, British Pharmacopoeia (bp) Reference Standard
212. Amitriptyline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
213. Amitriptyline Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
214. Amitriptyline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
215. 1-propanamine, 3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-ylidene)-n,n-dimethyl-, Hydrochloride (1:1)
216. 10,11-dihydro-n,n-dimethyl-5h-dibenzo[a,d]cycloheptene-delta5,gamma-propylamine Hydrochloride
217. 3-(10,11-dihydro-5h-dibenzo[a,d]cyc Lohepten-5-ylidene)-n,n-dimethyl-1-propanamine Hydrochloride
218. 5h-dibenzo[a,d]cycloheptene-delta(sup5),gamma-propylamine, 10,11-dihydro-n,n-dimethyl-, Hydrochloride
219. 5h-dibenzo[a,d]cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-n,n-dimethyl-, Hydrochloride (6ci,8ci)
220. Amitriptyline Hydrochloride Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
221. N,n-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,11,13-hexaenylidene)propan-1-amine;hydron;chloride
| Molecular Weight | 313.9 g/mol |
|---|---|
| Molecular Formula | C20H24ClN |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 313.1597275 g/mol |
| Monoisotopic Mass | 313.1597275 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Amitriptyline hydrochloride |
| Drug Label | DESCRIPTIONAmitriptyline HCl is 3-(10,11-dihydro-5H-dibenzo [a,d] cycloheptene-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride. Its empirical formula is C20H23NHCl, and its structural formula isAmitriptyline HCl, a dibenzocycloheptadiene deriv... |
| Active Ingredient | Amitriptyline hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 75mg; 100mg; 25mg; 50mg; 10mg; 150mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Mutual Pharm; Sun Pharm Inds; Sandoz; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Amitriptyline hydrochloride |
| Drug Label | DESCRIPTIONAmitriptyline HCl is 3-(10,11-dihydro-5H-dibenzo [a,d] cycloheptene-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride. Its empirical formula is C20H23NHCl, and its structural formula isAmitriptyline HCl, a dibenzocycloheptadiene deriv... |
| Active Ingredient | Amitriptyline hydrochloride |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 75mg; 100mg; 25mg; 50mg; 10mg; 150mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Mutual Pharm; Sun Pharm Inds; Sandoz; Mylan |
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

Registration Number : 302MF10137
Registrant's Address : Plot No. 2G, 2H, 2I, Udyog Vihar, Greater Noida-201 308 (U.P.) India
Initial Date of Registration : 2020-11-26
Latest Date of Registration :
Date of Issue : 2022-06-22
Valid Till : 2025-05-20
Written Confirmation Number : WC-0001
Address of the Firm :

NDC Package Code : 15894-0033
Start Marketing Date : 2019-01-23
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (100kg/100kg)
Marketing Category : BULK INGREDIENT
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
Date of Issue : 2022-06-22
Valid Till : 2025-05-20
Written Confirmation Number : WC-001N
Address of the Firm :

GDUFA
DMF Review : Reviewed
Rev. Date : 2017-03-17
Pay. Date : 2017-03-02
DMF Number : 2719
Submission : 1976-08-09
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2413
Submission : 1975-03-10
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4311
Submission : 1981-10-19
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2908
Submission : 1977-03-18
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2413
Submission : 1975-03-10
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2535
Submission : 1975-10-06
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3449
Submission : 1979-02-26
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3008
Submission : 1977-07-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2598
Submission : 1976-01-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-03-17
Pay. Date : 2017-03-02
DMF Number : 2719
Submission : 1976-08-09
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11027
Submission : 1994-07-21
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7802
Submission : 1988-11-28
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01 (amitriptyline hydrochloride) is being evaluated in the mid-stage clinical trial studies for patients suffering from ChemoTherapy-induced peripheral neuropathy.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 18, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AlgoTx Reports Positive Phase 2 ATX01 Data in CIPN Treatment
Details : ATX01 (amitriptyline hydrochloride) is being evaluated in the mid-stage clinical trial studies for patients suffering from ChemoTherapy-induced peripheral neuropathy.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
February 18, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01 (amitriptyline hydrochloride) is being evaluated in the mid-stage clinical trial studies for patients suffering from ChemoTherapy-induced peripheral neuropathy.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AlgoTx "Last Patient Visit" in Ph II of ATX01 for Chemotherapy-Induced Neuropathy
Details : ATX01 (amitriptyline hydrochloride) is being evaluated in the mid-stage clinical trial studies for patients suffering from ChemoTherapy-induced peripheral neuropathy.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01 (amitriptyline hydrochloride) topical which is investigated in Phase 2 for pain of Erythromelalgia, a rare neurological and vascular disease characterized by painful extremities for which there is no available treatment. The trial is due to read out in H1 2024.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Rare Diseases and Disorders Brand Name: ATX01
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Rare Diseases and Disorders
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ATX01 (amitriptyline hydrochloride) topical which is investigated in Phase 2 for pain of Erythromelalgia, a rare neurological and vascular disease characterized by painful extremities for which there is no available treatment. The trial is due to read ou...
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The net proceedings will be used to demonstrate the clinical efficacy of ATX01 (amitriptyline hydrochloride) in Phase 2 in chemotherapy-induced peripheral neuropathy and erythromelalgia.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Relyens Innovation Santé
Deal Size: $21.2 million Upfront Cash: Undisclosed
Deal Type: Series B Financing March 14, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Relyens Innovation Santé
Deal Size : $21.2 million
Deal Type : Series B Financing
AlgoTx Raises €20M to Conduct ATX01’s Phase 2 Program in Peripheral Neuropathic Pain
Details : The net proceedings will be used to demonstrate the clinical efficacy of ATX01 (amitriptyline hydrochloride) in Phase 2 in chemotherapy-induced peripheral neuropathy and erythromelalgia.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
March 14, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
As part of the transaction, MedQuímica will acquire rights to nine products including Limbitrol, Melleril and Dalmadorm for Central Nervous System related conditions, Bacrocin, Glyquin, Solaquin, Oxipelle™ and Efurix as topical oncological treatments.
Lead Product(s): Chlordiazepoxide,Amitriptyline Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Limbitrol
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: MedQuímica Indústria Farmacêutica
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition November 28, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Chlordiazepoxide,Amitriptyline Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : MedQuímica Indústria Farmacêutica
Deal Size : Undisclosed
Deal Type : Acquisition
Lupin's Brazil Subsidiary Acquires 9 Brands From Bausch Health
Details : As part of the transaction, MedQuímica will acquire rights to nine products including Limbitrol, Melleril and Dalmadorm for Central Nervous System related conditions, Bacrocin, Glyquin, Solaquin, Oxipelle™ and Efurix as topical oncological treatments.
Product Name : Limbitrol
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
November 28, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01 is a novel and patented topical formulation of amitriptyline. Its non-systemic mode of action locally inhibits pain signaling by the skin’s nerve fibers whilst minimizing systemic penetration, thus avoiding unwanted toxicity.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 06, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AlgoTx’s ATX01 Granted Fast Track Designation by FDA for Chemotherapy-Induced Neuropathic Pain
Details : ATX01 is a novel and patented topical formulation of amitriptyline. Its non-systemic mode of action locally inhibits pain signaling by the skin’s nerve fibers whilst minimizing systemic penetration, thus avoiding unwanted toxicity.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 06, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01 is a novel, patented, topical formulation of amitriptyline. Its non-systemic mode of action locally inhibits pain signaling in the skin’s nerve fibers whilst minimizing systemic penetration, thus avoiding unwanted toxicity.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ATX01 is a novel, patented, topical formulation of amitriptyline. Its non-systemic mode of action locally inhibits pain signaling in the skin’s nerve fibers whilst minimizing systemic penetration, thus avoiding unwanted toxicity.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATX01, an innovative topical treatment for the pain of peripheral neuropathy successfully reached its safety and pharmacokinetics objectives in Phase I trial, clearing the way for Phase II development in Chemotherapy-Induced Peripheral Neuropathy.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: Phase IProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 15, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ATX01, an innovative topical treatment for the pain of peripheral neuropathy successfully reached its safety and pharmacokinetics objectives in Phase I trial, clearing the way for Phase II development in Chemotherapy-Induced Peripheral Neuropathy.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 15, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The ANDA approval is for Amitriptyline HCl Tablets USP, 10 mg, 25 mg, 50 mg, 75 mg, 100 mg and 150 mg from the USFDA to market a generic version of ELAVIL (Amitriptyline Hydrochloride) 10 mg, 25 mg, 50 mg, 75 mg, 100 mg and 150 mg of AstraZeneca Pharmaceuticals LP.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 20, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
ANDA Approval from USFDA for Unichem’s Amitriptyline HCl Tablets USP, 10 mg, 25 mg, 50 mg, 75 mg...
Details : The ANDA approval is for Amitriptyline HCl Tablets USP, 10 mg, 25 mg, 50 mg, 75 mg, 100 mg and 150 mg from the USFDA to market a generic version of ELAVIL (Amitriptyline Hydrochloride) 10 mg, 25 mg, 50 mg, 75 mg, 100 mg and 150 mg of AstraZeneca Pharmac...
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 20, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AlgoTherapeutix has received approval from the ethics committee and the regulatory authority in the Czech Republic to initiate a Phase 1 clinical trial with ATX01.
Lead Product(s): Amitriptyline Hydrochloride
Therapeutic Area: Neurology Brand Name: ATX01
Study Phase: IND EnablingProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 15, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Amitriptyline Hydrochloride
Therapeutic Area : Neurology
Highest Development Status : IND Enabling
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
AlgoTherapeutix Receives Regulatory Approval to Initiate Clinical Development of ATX01
Details : AlgoTherapeutix has received approval from the ethics committee and the regulatory authority in the Czech Republic to initiate a Phase 1 clinical trial with ATX01.
Product Name : ATX01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 15, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 1210-35-1
End Use API : Amitriptyline Hydrochloride
About The Company : Tagoor Laboratories, established in 2018, is a part of the Tagoor Group. It specializes in providing APIs, advanced intermediates and key starting materials for...
3-Dimethylaminopropyl chloride hydrochloride
CAS Number : 5407-04-5
End Use API : Amitriptyline Hydrochloride
About The Company : Arran is an independent fine chemical company located near Athlone, Ireland. Our range of pharmaceutical intermediates includes alkylamines and hydrochlorides, ...

3-Dimethylamino-1-propyl chloride hydrochloride
CAS Number : 5407-04-5
End Use API : Amitriptyline Hydrochloride
About The Company : Corey Organics is focused in developing and manufacturing of Active Pharmaceutical Ingredients (APIs), registered Pharma Intermediates, Fine Chemicals and Pyrid...

3-(Dimethylamino) Propylchloride HCI (DMPC)
CAS Number : 5407-04-5
End Use API : Amitriptyline Hydrochloride
About The Company : Darshan Pharmachem, founded in 2011 in Ankleshwar, is a leading pharmaceutical company specializing in high-quality and innovative API intermediates. We are com...

CAS Number : 1210-35-1
End Use API : Amitriptyline Hydrochloride
About The Company : Established in 1984, R L Fine Chem Pvt. Ltd. is one of the fastest growing API companies, with a leadership position in several APIs such as antihistamines, ant...

CAS Number : 1210-35-1
End Use API : Amitriptyline Hydrochloride
About The Company : VASUDHA PHARMA CHEM LIMITED was incorporated, as a public limited company under the Companies Act, 1956 in 1994-95 at Hyderabad in the state of Telangana, India...

3-(N,N Di methyl amino) propylchloride HCl
CAS Number : 1281001
End Use API : Amitriptyline Hydrochloride
About The Company : VASUDHA PHARMA CHEM LIMITED was incorporated, as a public limited company under the Companies Act, 1956 in 1994-95 at Hyderabad in the state of Telangana, India...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 10mg
Packaging : Pack Size 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 10x10
Regulatory Info :
Dosage : Tablet
Dosage Strength : 10mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 25mg
Packaging : Pack Size 10x10
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging : Pack Size 10x10
Regulatory Info :
Dosage : Tablet
Dosage Strength : 25mg
Brand Name :
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 10mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 10mg/ml
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Amitriptyline Hydrochl...
Dosage Form : DC Granules
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Packaging :
Regulatory Info :
Dosage : DC Granules
Dosage Strength : 25MG
Brand Name : Amitriptyline Hydrochl...
Approval Date :
Application Number :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Amitriptyline Hydrochl...
Dosage Form : DC Granules
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Packaging :
Regulatory Info :
Dosage : DC Granules
Dosage Strength : 50MG
Brand Name : Amitriptyline Hydrochl...
Approval Date :
Application Number :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Regulatory Info :
Registration Country : India
Brand Name : Amitriptyline Hydrochl...
Dosage Form : DC Granules
Dosage Strength : 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius Pharma has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Packaging :
Regulatory Info :
Dosage : DC Granules
Dosage Strength : 100MG
Brand Name : Amitriptyline Hydrochl...
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Spain
Brand Name : DEPRELIO
Dosage Form : Capsule
Dosage Strength : 25MG
Packaging : Blister (PVC / Aluminum) with 30 Capsules.
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Blister (PVC / Aluminum) with 30 Capsules.
Regulatory Info :
Dosage : Capsule
Dosage Strength : 25MG
Brand Name : DEPRELIO
Approval Date :
Application Number :
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : DC Granules
Dosage Strength : 100MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : DC Granules
Dosage Strength : 100MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : RELIDEP -50
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 50MG
Brand Name : RELIDEP -50
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : DC Granules and Tablet...
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : DC Granules and Tablet...
Dosage Strength : 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgel Capsule, Softgels
Grade : Parenteral, Topical, Oral
Category : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Brand Name : Polyethylene Glycol 400
Application : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : PEG 400 is used as a suspending agent, stabilizer, plasticizer and filler in OSDs, liquids & semi-solids and as a solvent for parenteral formulations.
Dosage Form : Capsule, Tablet, Topical Film, Transdermal Patch
Grade : Not Available
Category : Controlled & Modified Release, Direct Compression, Granulation
Application : Controlled & Modified Release, Direct Compression, Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Lauryl Sulfate
Application : Controlled & Modified Release
Excipient Details : PLLA-PEG used in the synthesis of targeted nanoparticles which are used for differential delivery and controlled release of drugs.
Pharmacopoeia Ref : NA
Technical Specs : Nano-particles, ultrapure, low-monomer & powder grades
Ingredient(s) : Poly L Lactide
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Granule / Pellet, Tablet
Grade : Oral & Topical
Category : Controlled & Modified Release, Lubricants & Glidants
Application : Controlled & Modified Release, Lubricants & Glidants
Excipient Details : Talc is a widely used as a dissolution retardant in the development of controlled release products. Talc is also used as a lubricant in tablet formulations.
Application : Controlled & Modified Release
Excipient Details : PLGA-PEG is used in the synthesis of targeted nanoparticles which are used for differential delivery and controlled release of drugs.
Pharmacopoeia Ref : NA
Technical Specs : Nano-particles, ultrapure, low-monomer & powder grades.
Ingredient(s) : Poly-DL-Lactic-co-Glycolic Acid
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Sustained Release Tablet Matrix
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Capsule, Cream / Lotion / Ointment, Gel, Tablet
Grade : Topical and Oral
Category : Controlled & Modified Release, Topical
Brand Name : Polyethylene Glycol 400
Application : Controlled & Modified Release, Topical
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : polyethylene glycol
Dosage Form : Tablet
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Starch
Application : Solubilizers
Excipient Details : HPC-SSL SFP is used as a binder and solubilizer in solid dosage forms including tablets, capsules, granules, and pellets.
Pharmacopoeia Ref : EP, JP, USP/NF, CEP, GMP, Hala...
Technical Specs : N/A
Ingredient(s) : Hydroxypropyl Cellulose
Dosage Form : Emulsion, Injectable / Parenteral
Grade : Parenteral
Category : Emulsifying Agents, Parenteral, Solubilizers
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Injectable / Parenteral, Suspension, Tablet
Grade : Parenteral, Oral, Topical
Category : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Application : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : Polysorbate 80 is used as a plasticizer, solubilizer, emulsifier, surfactant, and suspension stabilizer. It is also used in parenteral products.
Dosage Form : Capsule, Cream / Lotion / Ointment, Suspension, Tablet
Grade : Oral, Topical & Parenteral
Category : Solubilizers, Surfactant & Foaming Agents
Application : Solubilizers, Surfactant & Foaming Agents
Excipient Details : Polysorbate 80 acts as solubilizer, emulsifier and wetting agent.
Dosage Form : Gel, Softgel Capsule, Solution, Suppository
Grade : Not Available
Category : Solubilizers
Application : Solubilizers
Excipient Details : Nonionic solubilizer, emulsifier and co-emulsifier
Brand Name : AFFINISOL HPMC HME
Application : Solubilizers
Excipient Details : Solubility enhancement, Spray-Dried Dispersion (SDD), Hot Melt Extrusion (HME)
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Paste, Shampoo, Solution, Syrup, Tablet
Grade : Topical, Oral
Category : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Film Formers & Plasticizers, Solubilizers, Surfactant & Foaming Agents, Topical
Excipient Details : Non-Ionic Hydrophilic Surfactant, Emulsifier, Solubilizer
Pharmacopoeia Ref : Ph.Eur, USP-NF
Technical Specs : HLB: 15, EO: 20; EXCiPACT
Ingredient(s) : Polysorbate 80
Brand Name : Polysorbate 80 Multi-Compendial
Application : Solubilizers
Excipient Details : A & C's Polysorbate 80 multi-compendial is an excipient which meets USP-NF, EP, BP and JP monographs.
Brand Name : Microlex® PVD K30
Application : Solubilizers
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Also Available as Microlex® PVD K90.
Ingredient(s) : Povidone
Brand Name : Polysorbate 80 NF
Application : Solubilizers
Excipient Details : A & C's Polysorbate 80 is an excipient which meets the NF monograph.
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
44
PharmaCompass offers a list of Amitriptyline Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amitriptyline Hydrochloride manufacturer or Amitriptyline Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amitriptyline Hydrochloride manufacturer or Amitriptyline Hydrochloride supplier.
PharmaCompass also assists you with knowing the Amitriptyline Hydrochloride API Price utilized in the formulation of products. Amitriptyline Hydrochloride API Price is not always fixed or binding as the Amitriptyline Hydrochloride Price is obtained through a variety of data sources. The Amitriptyline Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sarotena manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sarotena, including repackagers and relabelers. The FDA regulates Sarotena manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sarotena API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sarotena manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sarotena supplier is an individual or a company that provides Sarotena active pharmaceutical ingredient (API) or Sarotena finished formulations upon request. The Sarotena suppliers may include Sarotena API manufacturers, exporters, distributors and traders.
click here to find a list of Sarotena suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sarotena DMF (Drug Master File) is a document detailing the whole manufacturing process of Sarotena active pharmaceutical ingredient (API) in detail. Different forms of Sarotena DMFs exist exist since differing nations have different regulations, such as Sarotena USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sarotena DMF submitted to regulatory agencies in the US is known as a USDMF. Sarotena USDMF includes data on Sarotena's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sarotena USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sarotena suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sarotena Drug Master File in Japan (Sarotena JDMF) empowers Sarotena API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sarotena JDMF during the approval evaluation for pharmaceutical products. At the time of Sarotena JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sarotena suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Sarotena Drug Master File in Korea (Sarotena KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Sarotena. The MFDS reviews the Sarotena KDMF as part of the drug registration process and uses the information provided in the Sarotena KDMF to evaluate the safety and efficacy of the drug.
After submitting a Sarotena KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Sarotena API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Sarotena suppliers with KDMF on PharmaCompass.
A Sarotena CEP of the European Pharmacopoeia monograph is often referred to as a Sarotena Certificate of Suitability (COS). The purpose of a Sarotena CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Sarotena EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Sarotena to their clients by showing that a Sarotena CEP has been issued for it. The manufacturer submits a Sarotena CEP (COS) as part of the market authorization procedure, and it takes on the role of a Sarotena CEP holder for the record. Additionally, the data presented in the Sarotena CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Sarotena DMF.
A Sarotena CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Sarotena CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Sarotena suppliers with CEP (COS) on PharmaCompass.
A Sarotena written confirmation (Sarotena WC) is an official document issued by a regulatory agency to a Sarotena manufacturer, verifying that the manufacturing facility of a Sarotena active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sarotena APIs or Sarotena finished pharmaceutical products to another nation, regulatory agencies frequently require a Sarotena WC (written confirmation) as part of the regulatory process.
click here to find a list of Sarotena suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Sarotena as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Sarotena API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Sarotena as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Sarotena and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Sarotena NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Sarotena suppliers with NDC on PharmaCompass.
Sarotena Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sarotena GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sarotena GMP manufacturer or Sarotena GMP API supplier for your needs.
A Sarotena CoA (Certificate of Analysis) is a formal document that attests to Sarotena's compliance with Sarotena specifications and serves as a tool for batch-level quality control.
Sarotena CoA mostly includes findings from lab analyses of a specific batch. For each Sarotena CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sarotena may be tested according to a variety of international standards, such as European Pharmacopoeia (Sarotena EP), Sarotena JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sarotena USP).
