
Synopsis
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Phxa34
2. Phxa41
3. Xalatan
1. 130209-82-4
2. Xalatan
3. Phxa41
4. Phxa-41
5. Xa41
6. Phxa 41
7. Xa-41
8. Latanoprost (isopropyl Ester)
9. Isopropyl (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((3r)-3-hydroxy-5-phenylpentyl)cyclopentyl)-5-heptenoate
10. Propan-2-yl (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl]hept-5-enoate
11. Latanoprost, (+/-)-
12. 6z5b6hvf6o
13. 8s5fb3xxg8
14. Latanoprost, Ethanol Solution
15. Chebi:6384
16. Propan-2-yl (5z)-7-{(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl}hept-5-enoate
17. T-2345
18. T2345
19. 5-heptenoic Acid, 7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl]-, 1-methylethyl Ester, (5z)-
20. Isopropyl (5z,9alpha,11alpha,15r)-9,11,15-trihydroxy-17-phenyl-18,19,20-trinorprost-5-en-1-oate
21. Catioprost
22. (z)-isopropyl 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((r)-3-hydroxy-5-phenylpentyl)cyclopentyl)hept-5-enoate
23. 155551-81-8
24. 5-heptenoic Acid, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenylpentyl)cyclopentyl)-, 1-methylethyl Ester
25. Smr000466354
26. Xalatan (tn)
27. Unii-6z5b6hvf6o
28. Latanoprostum
29. Nova-21027
30. Latanoprost [usan:inn:ban]
31. Xa 41
32. Phxa34 [as 15(r,s)-isomer]
33. Propan-2-yl (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl]hept-5-enoate
34. Ar-202
35. Mfcd00216074
36. Xelpros
37. L-ppds
38. Latanoprost [mi]
39. Latanoprost [inn]
40. Latanoprost [jan]
41. Latanoprost [usan]
42. Unii-8s5fb3xxg8
43. Latanoprost [vandf]
44. Chembl1051
45. Latanoprost [mart.]
46. Schembl24698
47. Latanoprost [usp-rs]
48. Latanoprost [who-dd]
49. Mls000759468
50. Mls001424106
51. Latanoprost (jan/usp/inn)
52. Gtpl1961
53. Dtxsid1041057
54. Latanoprost [orange Book]
55. Hms2051h11
56. Hms2089j17
57. Hms3715n22
58. Latanoprost [ep Monograph]
59. Latanoprost [usp Monograph]
60. Amy30089
61. Ex-a1770
62. Hy-b0577
63. Xalacom Component Latanoprost
64. Bdbm50240648
65. S4709
66. Zinc12468792
67. Latanoprost, >=98% (hplc), Oil
68. Rocklatan Component Latanoprost
69. Akos024458331
70. Ccg-100946
71. Db00654
72. Nc00196
73. Latanoprost Component Of Rocklatan
74. Ncgc00246969-01
75. Ncgc00246969-06
76. (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptanoic Acid 1-methylethyl Ester
77. As-75099
78. L0262
79. D00356
80. Ab00640005-04
81. Ab00640005-06
82. 209l824
83. A806039
84. Q634959
85. Sr-01000759428
86. J-005764
87. Sr-01000759428-4
88. Latanoprost, United States Pharmacopeia (usp) Reference Standard
89. Tris(2,4-dimethylphenyl)phosphine-5,5',5""""-trisulfonic Acid Trisodium Salt
90. (1r,2r,3r,5s,3''r)-7-[3,5-dihydroxy-2-(3-hydroxy-5-phenyl-pentyl)-cyclopentyl]-hept-5-enoic Acid Isopropyl Ester
91. (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(3r)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoic Acid Propan-2-yl Ester
92. 5-heptenoic Acid, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenylpentyl)cyclopentyl)-, 1-methylethyl Ester, (1r-(1-alpha(z),2-beta(r*),3-alpha,5-alpha))-
93. 5-heptenoic Acid, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenylpentyl)cyclopentyl)-1-methylethyl Ester, (1r-(1.alpha.(z),2.beta.(r*),3.alpha.,5.alpha.))-
94. Isopropyl (5z,9alpha,11alpha,15r)-9,11,15-trihydroxy-17-phenyl-18,19,20-trinor-prost-5-en-1-oate;xalatan
95. Propan-2-yl (z)-7-[(1r,2r,3r,5s)-3,5-bis(oxidanyl)-2-[(3r)-3-oxidanyl-5-phenyl-pentyl]cyclopentyl]hept-5-enoate
| Molecular Weight | 432.6 g/mol |
|---|---|
| Molecular Formula | C26H40O5 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 14 |
| Exact Mass | 432.28757437 g/mol |
| Monoisotopic Mass | 432.28757437 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 526 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Latanoprost |
| PubMed Health | Latanoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
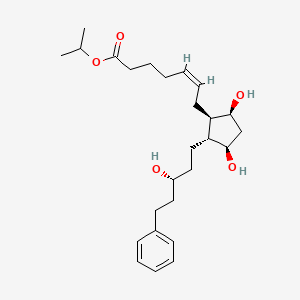
| Drug Label | Latanoprost is a prostaglandin F2 analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:Latanoprost is a... |
| Active Ingredient | Latanoprost |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.005% |
| Market Status | Tentative Approval; Prescription |
| Company | Alcon Res; Par Pharm; Bausch And Lomb; Luitpold; Dr Reddys Labs; Mylan; Akorn |
| 2 of 4 | |
|---|---|
| Drug Name | Xalatan |
| PubMed Health | Latanoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Latanoprost is a prostaglandin F2 analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:Latanoprost is a... |
| Active Ingredient | Latanoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 3 of 4 | |
|---|---|
| Drug Name | Latanoprost |
| PubMed Health | Latanoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Latanoprost is a prostaglandin F2 analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:Latanoprost is a... |
| Active Ingredient | Latanoprost |
| Dosage Form | Solution/drops; Solution |
| Route | Ophthalmic; ophthalmic |
| Strength | 0.005% |
| Market Status | Tentative Approval; Prescription |
| Company | Alcon Res; Par Pharm; Bausch And Lomb; Luitpold; Dr Reddys Labs; Mylan; Akorn |
| 4 of 4 | |
|---|---|
| Drug Name | Xalatan |
| PubMed Health | Latanoprost (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | Latanoprost is a prostaglandin F2 analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:Latanoprost is a... |
| Active Ingredient | Latanoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.005% |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Latanoprost is indicated for the reduction of elevated intraocular pressure in patients who have been diagnosed with open-angle glaucoma or ocular hypertension. Latanoprost may be combined in a product with [Netarsudil], a rho kinase inhibitor, for the same indications. In addition to the above indications, the Canadian monograph for this drug also approves latanoprost for the treatment of elevated intraocular pressure as a result of angle-closure glaucoma that has been treated with peripheral iridotomy or laser iridoplasty.
FDA Label
Treatment of glaucoma
Latanoprost effectively decreases intraocular pressure by increasing uveoscleral outflow. A decrease in intraocular pressure has been measured within 34 hours post-administration, reaches a maximum decrease at 812 hours, and can be maintained for a period of 24 hours. **A note on eye and periorbital changes** Between 3 to 10% of patients taking latanoprost have experienced iris pigmentation after about 3-4 months of latanoprost use. Patients should be notified of this risk before initiating treatment. It may occur in both patients with light-colored irides (green-brown or blue/grey-brown) or dark-colored (brown) irides, but is less pronounced in the latter group. This drug may also cause other ocular effects including infrequent conjunctival hyperemia, pigmentation of periocular tissues, eyelash changes, hypertrichosis, and ocular irritation.
Ophthalmic Solutions
Sterile solutions that are intended for instillation into the eye. It does not include solutions for cleaning eyeglasses or CONTACT LENS SOLUTIONS. (See all compounds classified as Ophthalmic Solutions.)
S01EE01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EE - Prostaglandin analogues
S01EE01 - Latanoprost
Absorption
This drug is rapidly absorbed in the cornea as an isopropyl ester prodrug and is then activated by the process of hydrolysis. A small amount of this drug is systemically absorbed. The Cmax of latanoprost in the systemic circulation is reached after 5 minutes and is measured to be 53 pg/mL. The Cmax in the aqueous humor is attained within 2 hours after administration. and has been estimated to be 15-30 ng/mL.
Route of Elimination
After hepatic beta-oxidation, the metabolites of latanoprost are primarily found to be excreted by the kidneys. About 88% of the latanoprost dose is recovered in the urine after topical administration. About 15% of a dose is reported to be excreted in the feces.
Volume of Distribution
The volume of distribution of latanoprost is 0.16 0.02 L/kg. The activated acid form of latanoprost can be measured in aqueous humor in the initial 4 hours post-administration, and it is measured in the plasma only for 1 hour following ophthalmic administration. This drug is more lipophilic than its parent prostaglandin and easily penetrates the cornea. It has been shown to cross the placenta in rats.
Clearance
The systemic clearance of latanoprost is 7 mL/min/kg.
After corneal uptake, this prodrug is hydrolyzed and activated by esterases to become a pharmacologically active drug. The small portion of this drug that is able to reach the circulation is found to be metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites through fatty acid beta-oxidation.
The elimination half-life of latanoprost from the plasma is about 17 minutes. The elimination half-life of latanoprost from the eye is estimated at 23 hours.
Elevated intraocular pressure leads to an increased risk of glaucomatous visual field loss. The higher the intraocular pressure, the higher the risk of damage to the optic nerve and loss of visual field. Latanoprost selectively stimulates the prostaglandin F2 alpha receptor and this results in a decreased intraocular pressure (IOP) via the increased outflow of aqueous humor, which is often implicated in cases of elevated intraocular pressure. Possible specific mechanisms of the abovementioned increased aqueous outflow are the remodeling of the extracellular matrix and regulation of matrix metalloproteinases. These actions result in higher tissue permeability related to humor outflow pathways, which likely change outflow resistance and/or outflow rates.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2017-03-17
Pay. Date : 2016-11-10
DMF Number : 26081
Submission : 2012-05-14
Status : Active
Type : II

Registration Number : 302MF10040
Registrant's Address : ul. Pra(´)ce, 657, 277 11 Neratovice, Czech Republic
Initial Date of Registration : 2020-04-02
Latest Date of Registration :

Registrant Name : Eltek Pharmachem Co., Ltd.
Registration Date : 2022-01-24
Registration Number : 20220124-211-J-1221
Manufacturer Name : Cayman Pharma sro
Manufacturer Address : ul. Práce 657 277 11 Neratovice

| Available Reg Filing : CA, ASMF |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18343
Submission : 2005-05-24
Status : Active
Type : II

Certificate Number : CEP 2021-379 - Rev 01
Issue Date : 2024-08-21
Type : Chemical
Substance Number : 2230
Status : Valid

Registration Number : 229MF10209
Registrant's Address : No. 2, Shih 4th Rd. , Yangmei Dist. , Taoyuan City 326013, Taiwan
Initial Date of Registration : 2017-12-01
Latest Date of Registration :

Registrant Name : Korea Santen Pharmaceutical Co., Ltd.
Registration Date : 2023-11-20
Registration Number : 20231120-211-J-1567
Manufacturer Name : Chirogate International Inc. Wulin Plant
Manufacturer Address : No. 41, Sec. 1, Gong 2nd Rd., and No. 22, Alley 39, Sec. 1, Gong 2nd Rd., Longtan Dist., Taoyuan City 325019, Taiwan
 Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-09-11
Pay. Date : 2013-01-16
DMF Number : 26728
Submission : 2012-12-14
Status : Active
Type : II

Certificate Number : R0-CEP 2021-352 - Rev 00
Issue Date : 2022-12-02
Type : Chemical
Substance Number : 2230
Status : Valid

Registration Number : 225MF10111
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2013-05-30
Latest Date of Registration :

Registrant Name : Samil Pharmaceutical Co., Ltd.
Registration Date : 2022-10-18
Registration Number : 20221018-211-J-1382
Manufacturer Name : EUROAPI Hungary Ltd.
Manufacturer Address : To utca 1-5., Budapest, 1045, Hungary

| Available Reg Filing : ASMF |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-09-10
Pay. Date : 2013-08-16
DMF Number : 17445
Submission : 2004-06-01
Status : Active
Type : II

Certificate Number : CEP 2022-093 - Rev 01
Issue Date : 2024-11-25
Type : Chemical
Substance Number : 2230
Status : Valid

Registration Number : 221MF10182
Registrant's Address : 1180 E. Ellsworth Road, Ann Arbor, MI 48108, USA
Initial Date of Registration : 2009-08-13
Latest Date of Registration :
 Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-10-15
Pay. Date : 2021-06-29
DMF Number : 34369
Submission : 2020-01-27
Status : Active
Type : II

Certificate Number : R0-CEP 2021-059 - Rev 00
Issue Date : 2023-07-26
Type : Chemical
Substance Number : 2230
Status : Valid

| Available Reg Filing : ASMF |
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2017-03-17
Pay. Date : 2016-11-10
DMF Number : 26081
Submission : 2012-05-14
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2017-04-13
Pay. Date : 2017-02-21
DMF Number : 31372
Submission : 2017-03-14
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18343
Submission : 2005-05-24
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-09-11
Pay. Date : 2013-01-16
DMF Number : 26728
Submission : 2012-12-14
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-09-10
Pay. Date : 2013-08-16
DMF Number : 17445
Submission : 2004-06-01
Status : Active
Type : II
 Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
GDUFA
DMF Review : Complete
Rev. Date : 2021-10-15
Pay. Date : 2021-06-29
DMF Number : 34369
Submission : 2020-01-27
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : Complete
Rev. Date : 2012-12-26
Pay. Date : 2012-12-03
DMF Number : 22198
Submission : 2008-11-14
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19535
Submission : 2006-06-20
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15462
Submission : 2001-05-31
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20925
Submission : 2007-10-05
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Cayman Pharma, a leading European provider of cGMP prostaglandin APIs, was formed in 2006 through the merger of Cayman Chemical and NeraPharm, both with vast expertise in prostagla...
About the Company : Chirogate, established in 1999, is a leading supplier of niche-market molecules, specializing in prostaglandins. With a focus on quality and compliance, Chirogate has built a reput...
 Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
About the Company : Century Pharmaceuticals, established in 1982, has 40 years of experience in manufacturing APIs. It has been supplying APIs produced in-house to several major pharma companies in In...
About the Company : EUROAPI is focused on reinventing active ingredient solutions to meet the needs of customers and patients worldwide sustainably. We are a leading player in APIs with approximately ...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
Gentec Pharmaceutical Group is focused on manufacturing & developing APIs/HPAPIs, Advanced Intermediates & Fine Chemicals.
About the Company : With more than 30 years of experience, Gentec Pharmaceutical Group has established itself as one of the leaders in raw materials and ingredients for the food, dietary and nutrition...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
 Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
Capital Farma, a leading European pharmaceutical company focusing on the development & distribution of niche APIs & Pharma Services.
About the Company : Capital Farma offers a comprehensive range of pharmaceutical solutions. Our API offering includes active ingredients sourced exclusively from top European manufacturers, ensuring t...
About the Company : Monvi Laboratories PVT LTD, is a Research and Development Organization, headquartered in Hyderabad, India. We aspire to emerge as a global player in delivering high quality, innova...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 55444-68-3
End Use API : Latanoprost
About The Company : Ceyone Life Sciences specializes in providing research-oriented chemistry services and manufacturing niche chemicals, intermediates, and APIs. The company is fo...

CAS Number : 145667-75-0
End Use API : Latanoprost
About The Company : Established in August 2011, Raffles PharmaTech is a high-tech enterprise that focus on development, manufacturing and sales of high-value added active pharmaceu...

(3aR,4R,5R,6aS)-4-((R)-3-hydroxy-5-phenylpentyl)-2...
CAS Number : 353522-93-7
End Use API : Latanoprost
About The Company : Established in August 2011, Raffles PharmaTech is a high-tech enterprise that focus on development, manufacturing and sales of high-value added active pharmaceu...

CAS Number : 75-30-9
End Use API : Latanoprost
About The Company : At Infinium Pharmachem Pvt. Ltd., we are well focused to Iodine chemistry & this is the only core area of ours. We are working as CRAMS, but our domain is very ...

CAS Number : 145667-75-0
End Use API : Latanoprost
About The Company : Shenzhen HwaGen Pharmaceutical Co., Ltd. (HwaGen Pharma) was founded in December of 2015 under the leadership of a “National 1000 Talents Program” expert. B...

(3aR,4R,5R,6aS)-4-((R)-3-hydroxy-5-phenylpentyl)-2...
CAS Number : 353522-93-7
End Use API : Latanoprost
About The Company : Shenzhen HwaGen Pharmaceutical Co., Ltd. (HwaGen Pharma) was founded in December of 2015 under the leadership of a “National 1000 Talents Program” expert. B...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
45
PharmaCompass offers a list of Latanoprost API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Latanoprost manufacturer or Latanoprost supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Latanoprost manufacturer or Latanoprost supplier.
PharmaCompass also assists you with knowing the Latanoprost API Price utilized in the formulation of products. Latanoprost API Price is not always fixed or binding as the Latanoprost Price is obtained through a variety of data sources. The Latanoprost Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hysite manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hysite, including repackagers and relabelers. The FDA regulates Hysite manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hysite API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hysite manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hysite supplier is an individual or a company that provides Hysite active pharmaceutical ingredient (API) or Hysite finished formulations upon request. The Hysite suppliers may include Hysite API manufacturers, exporters, distributors and traders.
click here to find a list of Hysite suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hysite DMF (Drug Master File) is a document detailing the whole manufacturing process of Hysite active pharmaceutical ingredient (API) in detail. Different forms of Hysite DMFs exist exist since differing nations have different regulations, such as Hysite USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hysite DMF submitted to regulatory agencies in the US is known as a USDMF. Hysite USDMF includes data on Hysite's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hysite USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hysite suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Hysite Drug Master File in Japan (Hysite JDMF) empowers Hysite API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Hysite JDMF during the approval evaluation for pharmaceutical products. At the time of Hysite JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Hysite suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Hysite Drug Master File in Korea (Hysite KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Hysite. The MFDS reviews the Hysite KDMF as part of the drug registration process and uses the information provided in the Hysite KDMF to evaluate the safety and efficacy of the drug.
After submitting a Hysite KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Hysite API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Hysite suppliers with KDMF on PharmaCompass.
A Hysite CEP of the European Pharmacopoeia monograph is often referred to as a Hysite Certificate of Suitability (COS). The purpose of a Hysite CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hysite EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hysite to their clients by showing that a Hysite CEP has been issued for it. The manufacturer submits a Hysite CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hysite CEP holder for the record. Additionally, the data presented in the Hysite CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hysite DMF.
A Hysite CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hysite CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hysite suppliers with CEP (COS) on PharmaCompass.
A Hysite written confirmation (Hysite WC) is an official document issued by a regulatory agency to a Hysite manufacturer, verifying that the manufacturing facility of a Hysite active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hysite APIs or Hysite finished pharmaceutical products to another nation, regulatory agencies frequently require a Hysite WC (written confirmation) as part of the regulatory process.
click here to find a list of Hysite suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hysite as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hysite API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hysite as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hysite and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hysite NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hysite suppliers with NDC on PharmaCompass.
Hysite Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hysite GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hysite GMP manufacturer or Hysite GMP API supplier for your needs.
A Hysite CoA (Certificate of Analysis) is a formal document that attests to Hysite's compliance with Hysite specifications and serves as a tool for batch-level quality control.
Hysite CoA mostly includes findings from lab analyses of a specific batch. For each Hysite CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hysite may be tested according to a variety of international standards, such as European Pharmacopoeia (Hysite EP), Hysite JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hysite USP).
