
Synopsis
Synopsis
0
KDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Errolon
2. Frusemid
3. Frusemide
4. Furanthril
5. Furantral
6. Furosemide Monohydrochloride
7. Furosemide Monosodium Salt
8. Fursemide
9. Fusid
10. Lasix
1. 54-31-9
2. Frusemide
3. Lasix
4. Furosemid
5. Furanthril
6. Errolon
7. Fusid
8. Aisemide
9. Beronald
10. Desdemin
11. Frusemin
12. Fuluvamide
13. Furanthryl
14. Furantril
15. Fursemide
16. Lowpstron
17. Macasirool
18. Prefemin
19. Rosemide
20. Trofurit
21. Fulsix
22. Furesis
23. Katlex
24. Lasilix
25. Radonna
26. Seguril
27. Transit
28. Lasex
29. Salix
30. Urex
31. Marsemide
32. Eutensin
33. Frusetic
34. Fursemid
35. Logirene
36. Oedemex
37. Promedes
38. Frusid
39. Lazix
40. Mirfat
41. Frusemid
42. Frusenex
43. Furanturil
44. Furosedon
45. Profemin
46. Urosemide
47. Aluzine
48. Diural
49. Dryptal
50. Impugan
51. Nicorol
52. Rusyde
53. Uremide
54. Uresix
55. Yidoli
56. Disal
57. Laxur
58. Urian
59. Apo-frusemide
60. Hydro-rapid
61. Anfuramaide
62. Arasemide
63. Bioretic
64. Disemide
65. Diurapid
66. Diurolasa
67. Diusemide
68. Durafurid
69. Fluidrol
70. Frusedan
71. Fuluvamine
72. Furobeta
73. Furodiurol
74. Furodrix
75. Furorese
76. Furosemidum
77. Furosemix
78. Furoside
79. Furosifar
80. Furovite
81. Fursemida
82. Hissuflux
83. Hydroled
84. Jenafusid
85. Lasiletten
86. Lowpston
87. Moilarorin
88. Novosemide
89. Protargen
90. Radisemide
91. Selectofur
92. Sigasalur
93. Spirofur
94. Synephron
95. Zafimida
96. Aldalix
97. Aquarid
98. Aquasin
99. Cetasix
100. Dirine
101. Discoid
102. Diurin
103. Diusil
104. Diuzol
105. Dranex
106. Edemid
107. Edenol
108. Endural
109. Farsix
110. Franyl
111. Frumex
112. Frumide
113. Frusema
114. Furetic
115. Furfan
116. Furmid
117. Furocot
118. Furomen
119. Furomex
120. Furosan
121. Furose
122. Furosix
123. Furoter
124. Fursol
125. Hydrex
126. Kofuzon
127. Kolkin
128. Kutrix
129. Lasemid
130. Liside
131. Luscek
132. Nelsix
133. Odemase
134. Odemex
135. Promide
136. Puresis
137. Radouna
138. Salinex
139. Salurex
140. Salurid
141. Uridon
142. Uritol
143. Aldic
144. Depix
145. Desal
146. Eliur
147. Fluss
148. Furex
149. Furix
150. Golan
151. Hydro
152. Nadis
153. Retep
154. Rosis
155. Vesix
156. Mita
157. Apo-furosemide
158. Furo-puren
159. Lasix Retard
160. Polysquall A
161. 4-chloro-n-furfuryl-5-sulfamoylanthranilic Acid
162. Less Diur
163. Neo-renal
164. Furo-basan
165. Furosemidu
166. Urex-m
167. Nephron
168. Furomide M.d.
169. Furosemida
170. 4-chloro-n-(2-furylmethyl)-5-sulfamoylanthranilic Acid
171. Lb 502
172. 5-(aminosulfonyl)-4-chloro-2-[(2-furylmethyl)amino]benzoic Acid
173. 2-furfurylamino-4-chloro-5-sulfamoylbenzoic Acid
174. Lb-502
175. Nci-c55936
176. Lasix (tn)
177. Benzoic Acid, 5-(aminosulfonyl)-4-chloro-2-[(2-furanylmethyl)amino]-
178. 4-chloro-2-[(furan-2-ylmethyl)amino]-5-sulfamoylbenzoic Acid
179. Diumide-k
180. 4-chloro-5-sulfamoyl-n-furfuryl-anthranilic Acid
181. 4-chloro-2-(furan-2-ylmethylamino)-5-sulfamoylbenzoic Acid
182. Chlor-n-(2-furylmethyl)-5-sulfamylanthranilsaeure
183. Anthranilic Acid, 4-chloro-n-furfuryl-5-sulfamoyl-
184. Chembl35
185. 4-chloro-2-((furan-2-ylmethyl)amino)-5-sulfamoylbenzoic Acid
186. Nsc-269420
187. Benzoic Acid, 5-(aminosulfonyl)-4-chloro-2-((2-furanylmethyl)amino)-
188. 7lxu5n7zo5
189. Chebi:47426
190. Furosemidu [polish]
191. 5-(aminosulfonyl)-4-chloro-2-((2-furanylmethyl)amino)benzoic Acid
192. 5-(aminosulfonyl)-4-chloro-2-[(2-furanylmethyl)amino]benzoic Acid
193. Cas-54-31-9
194. Furosemidum [inn-latin]
195. Furosemida [inn-spanish]
196. Ncgc00016241-06
197. Sal Diureticum
198. 4-chloro-2-{[(furan-2-yl)methyl]amino}-5-sulfamoylbenzoic Acid
199. Dsstox_cid_648
200. Dsstox_rid_75710
201. Dsstox_gsid_20648
202. Furosemide "mita"
203. Fun
204. Furosemide Mita
205. Furosemide Oral
206. 5-(aminosulfonyl)-4-chloro-2-([2-furanylmethyl]amino)benzoic Acid
207. 5-(aminosulfonyl)-4-chloro-2-[(furan-2-ylmethyl)amino]benzoic Acid
208. Smr000058202
209. Ccris 1951
210. Furosemide ''mita''
211. Hsdb 3086
212. Sr-01000765380
213. Einecs 200-203-6
214. Hoe-058a
215. Unii-7lxu5n7zo5
216. Nsc 269420
217. Brn 0840915
218. Neosemid
219. Zafurida
220. Chlor-n-(2-furylmethyl)-5-sulfamylanthranilsaeure [german]
221. 5-(aminosulfonyl)-4-chloro-2-((2-furylmethyl)amino)benzoic Acid
222. Furosemide, 4
223. Urex M
224. Furosemide (lasix)
225. Prestwick_752
226. Frumil (salt/mix)
227. Mfcd00010549
228. Furosemide [usan:usp:inn:ban:jan]
229. Spectrum_001100
230. 1z9y
231. 4-chloro-2-[(2-furylmethyl)amino]-5-sulfamoylbenzoic Acid
232. Furosemide [mi]
233. Furosemide [inn]
234. Furosemide [jan]
235. Prestwick0_000341
236. Prestwick1_000341
237. Prestwick2_000341
238. Prestwick3_000341
239. Spectrum2_001005
240. Spectrum3_000437
241. Spectrum4_000560
242. Spectrum5_000744
243. Furosemide [hsdb]
244. Furosemide [iarc]
245. Furosemide [usan]
246. F0182
247. Furosemide [vandf]
248. Upcmld-dp022
249. Furosemide [mart.]
250. Schembl9811
251. Furosemide [usp-rs]
252. Furosemide [who-dd]
253. Furosemide [who-ip]
254. Oprea1_667724
255. Bspbio_000401
256. Bspbio_002054
257. Kbiogr_001259
258. Kbioss_001580
259. 5-18-09-00555 (beilstein Handbook Reference)
260. Mls001066374
261. Mls001306403
262. Mls002548896
263. Bidd:gt0139
264. Divk1c_000575
265. Spectrum1500310
266. Spbio_001129
267. Spbio_002322
268. Bpbio1_000443
269. Furosemide (jp17/usp/inn)
270. Gtpl4839
271. Furosemide [green Book]
272. Dtxsid6020648
273. Furosemide [orange Book]
274. Upcmld-dp022:001
275. Bdbm25902
276. Hms501m17
277. Kbio1_000575
278. Kbio2_001580
279. Kbio2_004148
280. Kbio2_006716
281. Kbio3_001274
282. Zinc35804
283. 4-chloro-2-(2-furylmethylamino)-5-sulfamoyl-benzoic Acid
284. Furosemide [ep Monograph]
285. Ninds_000575
286. Furosemide [usp Monograph]
287. Hms1569e03
288. Hms1920b03
289. Hms2090k06
290. Hms2091h05
291. Hms2096e03
292. Hms2233h03
293. Hms3259m03
294. Hms3370j22
295. Hms3655e09
296. Hms3713e03
297. Hms3874g03
298. Pharmakon1600-01500310
299. Furosemide 1.0 Mg/ml In Methanol
300. Furosemidum [who-ip Latin]
301. Albb-019200
302. Hy-b0135
303. Tox21_110322
304. Tox21_202213
305. Tox21_302971
306. Bbl027780
307. Ccg-40223
308. Nsc269420
309. Nsc757039
310. Stk177334
311. Wln: T5oj B1mr Cg Fvq Dszw
312. Akos000266625
313. Furosemide 100 Microg/ml In Methanol
314. Tox21_110322_1
315. Bcp9000708
316. Cs-1915
317. Db00695
318. Ks-1226
319. Nc00453
320. Nsc-757039
321. Idi1_000575
322. Smp1_000129
323. Ncgc00016241-01
324. Ncgc00016241-02
325. Ncgc00016241-03
326. Ncgc00016241-04
327. Ncgc00016241-05
328. Ncgc00016241-07
329. Ncgc00016241-08
330. Ncgc00016241-10
331. Ncgc00016241-11
332. Ncgc00090893-01
333. Ncgc00090893-02
334. Ncgc00090893-03
335. Ncgc00090893-05
336. Ncgc00090893-06
337. Ncgc00256523-01
338. Ncgc00259762-01
339. Ac-11067
340. Bf166384
341. Bp-13261
342. Sbi-0051389.p003
343. Db-052536
344. Ab00052001
345. Sw196894-3
346. 54f319
347. C07017
348. D00331
349. D87719
350. Ab00052001-10
351. Ab00052001-11
352. Ab00052001_12
353. Ab00052001_13
354. A830094
355. Q388801
356. Sr-01000765380-2
357. Sr-01000765380-3
358. Sr-01000765380-7
359. Brd-k78010432-001-05-8
360. Brd-k78010432-001-10-8
361. Z275128584
362. 4-chloro-2-(2-furanylmethylamino)-5-sulfamoylbenzoic Acid
363. Furosemide, British Pharmacopoeia (bp) Reference Standard
364. Furosemide, European Pharmacopoeia (ep) Reference Standard
365. 4-chloranyl-2-(furan-2-ylmethylamino)-5-sulfamoyl-benzoic Acid
366. Furosemide, United States Pharmacopeia (usp) Reference Standard
367. 4-chloro-n-furfuryl-5-sulfamoylanthranilic Acid Furosemide
368. 4-chloro-n-furfuryl-5-sulfamoylanthranilic Acid??furosemide
369. 5-(aminosulfamyl)-4-chloro-2-[(2-furanylmethyl)amino]benzoic Acid
370. 5-(aminosulfonyl)-4-chloro-2-[(2-furylmethyl)amino]benzoic Acid #
371. 4-chloro-n-furfuryl-5-sulfamoylanthranilic Acid Pound>>furosemide
372. Furosemide, Pharmaceutical Secondary Standard; Certified Reference Material
373. Furosemide For Peak Identification, European Pharmacopoeia (ep) Reference Standard
374. Furosemide Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 330.74 g/mol |
|---|---|
| Molecular Formula | C12H11ClN2O5S |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 330.0077203 g/mol |
| Monoisotopic Mass | 330.0077203 g/mol |
| Topological Polar Surface Area | 131 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 481 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Furosemide |
| PubMed Health | Furosemide |
| Drug Classes | Cardiovascular Agent |
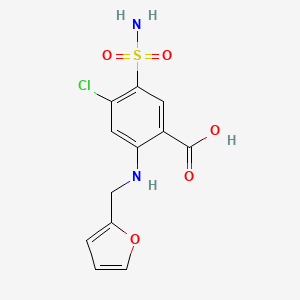
| Drug Label | The CAS Registry Number is 54-31-9.It has a molecular formula of C12H11ClN2O5S and a molecular weight of 330.75.The molecular structure is as follows:... |
| Active Ingredient | Furosemide |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 40mg/5ml; 10mg/ml; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Wockhardt; Excellium; Hospira; Sun Pharm Inds; Sandoz; Roxane; Ivax Sub Teva Pharms; Emcure Pharms; Fresenius Kabi Usa; Ipca Labs; Claris Lifesciences; Luitpold; Mylan |
| 2 of 6 | |
|---|---|
| Drug Name | Lasix |
| PubMed Health | Furosemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | LASIX is a diuretic which is an anthranilic acid derivative. LASIX tablets for oral administration contain furosemide as the active ingredient and the following inactive ingredients: lactose monohydrate NF, magnesium stearate NF, starch NF, talc US... |
| Active Ingredient | Furosemide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 3 of 6 | |
|---|---|
| Drug Name | Urex |
| Active Ingredient | Methenamine hippurate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1gm |
| Market Status | Prescription |
| Company | Cnty Line Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Furosemide |
| PubMed Health | Furosemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | The CAS Registry Number is 54-31-9.It has a molecular formula of C12H11ClN2O5S and a molecular weight of 330.75.The molecular structure is as follows:... |
| Active Ingredient | Furosemide |
| Dosage Form | Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 40mg/5ml; 10mg/ml; 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Wockhardt; Excellium; Hospira; Sun Pharm Inds; Sandoz; Roxane; Ivax Sub Teva Pharms; Emcure Pharms; Fresenius Kabi Usa; Ipca Labs; Claris Lifesciences; Luitpold; Mylan |
| 5 of 6 | |
|---|---|
| Drug Name | Lasix |
| PubMed Health | Furosemide |
| Drug Classes | Cardiovascular Agent |
| Drug Label | LASIX is a diuretic which is an anthranilic acid derivative. LASIX tablets for oral administration contain furosemide as the active ingredient and the following inactive ingredients: lactose monohydrate NF, magnesium stearate NF, starch NF, talc US... |
| Active Ingredient | Furosemide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 6 of 6 | |
|---|---|
| Drug Name | Urex |
| Active Ingredient | Methenamine hippurate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1gm |
| Market Status | Prescription |
| Company | Cnty Line Pharms |
Diuretics; Sodium Potassium Chloride Symporter Inhibitors
National Library of Medicine's Medical Subject Headings. Furosemide. Online file (MeSH, 2017). Available from, as of April 10, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
Oral Lasix may be used in adults for the treatment of hypertension alone or in combination with other antihypertensive agents. Hypertensive patients who cannot be adequately controlled with thiazides will probably also not be adequately controlled with Lasix alone. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Lasix (Furosemide Tablet) (Updated: April 2016). Available from, as of April 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c9b4d8f-0770-482d-a9e6-9c616a440b1a
Lasix is indicated in adults and pediatric patients for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome. Lasix is particularly useful when an agent with greater diuretic potential is desired. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Lasix (Furosemide Tablet) (Updated: April 2016). Available from, as of April 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c9b4d8f-0770-482d-a9e6-9c616a440b1a
IV furosemide has been found useful as an adjunct to hypotensive agents in the treatment of hypertensive crises, especially when associated with acute pulmonary edema or renal failure. In addition to producing a rapid diuresis, furosemide enhances the effects of other hypotensive drugs and counteracts the sodium retention caused by some of these agents. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2828
For more Therapeutic Uses (Complete) data for Furosemide (11 total), please visit the HSDB record page.
/BOXED WARNING/ Lasix (furosemide) is a potent diuretic which, if given in excessive amounts, can lead to a profound diuresis with water and electrolyte depletion. Therefore, careful medical supervision is required and dose and dose schedule must be adjusted to the individual patient's needs.
NIH; DailyMed. Current Medication Information for Lasix (Furosemide Tablet) (Updated: April 2016). Available from, as of April 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c9b4d8f-0770-482d-a9e6-9c616a440b1a
Excessive diuresis may cause dehydration and blood volume reduction with circulatory collapse and possibly vascular thrombosis and embolism, particularly in elderly patients. As with any effective diuretic, electrolyte depletion may occur during Lasix therapy, especially in patients receiving higher doses and a restricted salt intake. Hypokalemia may develop with Lasix, especially with brisk diuresis, inadequate oral electrolyte intake, when cirrhosis is present, or during concomitant use of corticosteroids, ACTH, licorice in large amounts, or prolonged use of laxatives. Digitalis therapy may exaggerate metabolic effects of hypokalemia, especially myocardial effects.
NIH; DailyMed. Current Medication Information for Lasix (Furosemide Tablet) (Updated: April 2016). Available from, as of April 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c9b4d8f-0770-482d-a9e6-9c616a440b1a
Patients receiving furosemide must be carefully observed for signs of hypovolemia, hyponatremia, hypokalemia, hypocalcemia, hypochloremia, and hypomagnesemia. Patients should be informed of the signs and symptoms of electrolyte imbalance and instructed to report to their physicians if weakness, dizziness, fatigue, faintness, mental confusion, lassitude, muscle cramps, headache, paresthesia, thirst, anorexia, nausea, and/or vomiting occur. Excessive fluid and electrolyte loss may be minimized by initiating therapy with small doses, careful dosage adjustment, using an intermittent dosage schedule if possible, and monitoring the patient's weight. To prevent hyponatremia and hypochloremia, intake of sodium may be liberalized in most patients; however, patients with cirrhosis usually require at least moderate sodium restriction while on diuretic therapy. Determinations of serum electrolytes, BUN, and carbon dioxide should be performed early in therapy with furosemide and periodically thereafter. If excessive diuresis and/or electrolyte abnormalities occur, the drug should be withdrawn or dosage reduced until homeostasis is restored. Electrolyte abnormalities should be corrected by appropriate measures.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2830
Furosemide should be used with caution in patients with hepatic cirrhosis because rapid alterations in fluid and electrolyte balance may precipitate hepatic precoma or coma.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2830
For more Drug Warnings (Complete) data for Furosemide (29 total), please visit the HSDB record page.
Furosemide is indicated for the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome, in adults and pediatric patients. Oral furosemide is indicated alone for the management of mild to moderate hypertension or severe hypertension in combination with other antihypertensive medications. Intravenous furosemide is indicated as adjunctive therapy in acute pulmonary edema when a rapid onset of diuresis is desired.
Treatment of fluid retention
Furosemide manages hypertension and edema associated with congestive heart failure, cirrhosis, and renal disease, including the nephrotic syndrome. Furosemide is a potent loop diuretic that works to increase the excretion of Na+ and water by the kidneys by inhibiting their reabsorption from the proximal and distal tubules, as well as the loop of Henle. It works directly acts on the cells of the nephron and indirectly modifies the content of the renal filtrate. Ultimately, furosemide increases the urine output by the kidney. Protein-bound furosemide is delivered to its site of action in the kidneys and secreted via active secretion by nonspecific organic transporters expressed at the luminal site of action. Following oral administration, the onset of the diuretic effect is about 1 and 1.5 hours, and the peak effect is reached within the first 2 hours. The duration of effect following oral administration is about 4-6 hours but may last up to 8 hours. Following intravenous administration, the onset of effect is within 5 minutes, and the peak effect is reached within 30 minutes. The duration of action following intravenous administration is approximately 2 hours. Following intramuscular administration, the onset of action is somewhat delayed.
Sodium Potassium Chloride Symporter Inhibitors
Agents that inhibit SODIUM-POTASSIUM-CHLORIDE SYMPORTERS which are concentrated in the thick ascending limb at the junction of the LOOP OF HENLE and KIDNEY TUBULES, DISTAL. They act as DIURETICS. Excess use is associated with HYPOKALEMIA and HYPERGLYCEMIA. (See all compounds classified as Sodium Potassium Chloride Symporter Inhibitors.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
C03CA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C03 - Diuretics
C03C - High-ceiling diuretics
C03CA - Sulfonamides, plain
C03CA01 - Furosemide
Absorption
Following oral administration, furosemide is absorbed from the gastrointestinal tract. It displays variable bioavailability from oral dosage forms, ranging from 10 to 90%. The oral bioavailability of furosemide from oral tablets or oral solution is about 64% and 60%, respectively, of that from an intravenous injection of the drug.
Route of Elimination
The kidneys are responsible for 85% of total furosemide total clearance, where about 43% of the drug undergoes renal excretion. Significantly more furosemide is excreted in urine following the I.V. injection than after the tablet or oral solution. Approximately 50% of the furosemide load is excreted unchanged in urine, and the rest is metabolized into glucuronide in the kidney.
Volume of Distribution
The volume of distribution following intravenous administration of 40 mg furosemide were 0.181 L/kg in healthy subjects and 0.140 L/kg in patients with heart failure.
Clearance
Following intravenous administration of 400 mg furosemide, the plasma clearance was 1.23 mL/kg/min in patients with heart failure and 2.34 mL/kg/min in healthy subjects, respectively.
Significantly more furosemide is excreted in urine following the IV injection than after the tablet or oral solution. There are no significant differences between the two oral formulations in the amount of unchanged drug excreted in urine.
NIH; DailyMed. Current Medication Information for Lasix (Furosemide Tablet) (Updated: April 2016). Available from, as of April 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c9b4d8f-0770-482d-a9e6-9c616a440b1a
After oral administration of furosemide to 18 pregnant women on the day of delivery, substantial concentrations of the drug were detected in umbilical cord vein plasma as well as in amniotic fluid. The ratio between the furosemide concentrations in maternal vein plasma and in umbilical cord plasma increased with time and approximated unity at 8 to 10 hr after administration of the drug. The plasma half-life of furosemide appeared to be longer in the mothers than in nonpregnant healthy volunteers. In one patient the plasma level of furosemide was constant during 5 hr of observation.
PMID:699480 Beermann B et al; Clin Pharmacol Ther 24 (5): 560-2 (1978)
In one study in patients with normal renal function, approx 60% of a single 80 mg oral dose of furosemide was absorbed from the GI tract. When admin to fasting adults in this dosage, the drug appeared in the serum within 10 min, reached a peak concn of 2.3 ug/mL in 60-70 min, & was almost completely cleared from the serum in 4 hr. When the same dose was given after a meal, the serum concn of furosemide increased slowly to a peak of about 1 ug/ml after 2 hr & similar concns were present 4 hr after ingestion. However, a similar diuretic response occurred regardless of whether the drug was given with food or to fasting patients. In another study, the rate & extent of absorption varied considerably when 1 g of furosemide was given orally to uremic patients. An avg of 76% of a dose was absorbed, & peak plasma concns were achieved within 2-9 hr (avg 4.4 hr). Serum concns required to produce max diuresis are not known, & it has been reported that the magnitude of response does not correlate with either the peak or the mean serum concns.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2831
The diuretic effect of orally administered furosemide is apparent within 30 minutes to 1 hr and is maximal in the first or second hour. The duration of action is usually 6-8 hr. The maximum hypotensive effect may not be apparent until several days after furosemide therapy is begun. After iv administration of furosemide, diuresis occurs within 5 min, reaches a maximum within 20-60 min, and persists for approximately 2 hr. After im administration, peak plasma concentrations are attained within 30 min; onset of diuresis occurs somewhat later than after iv administration. In patients with severely impaired renal function, the diuretic response may be prolonged.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2831
For more Absorption, Distribution and Excretion (Complete) data for Furosemide (15 total), please visit the HSDB record page.
The metabolism of furosemide occurs mainly in the kidneys and the liver, to a smaller extent. The kidneys are responsible for about 85% of total furosemide total clearance, where about 40% involves biotransformation. Two major metabolites of furosemide are furosemide glucuronide, which is pharmacologically active, and saluamine (CSA) or 4-chloro-5-sulfamoylanthranilic acid.
It would appear that frusemide glucuronide is the only or at least the major biotransformation metabolite in man. 2-amino-4- chloro-5-sulfamoylanthranilic acid has been reported in some studies but not in others; and is thought to be an analytical artifact.
International Programme on Chemical Safety (IPCS); Poisons Information Monograph (PIM) No. 240, Furosemide (July 1997). Available from, as of April 18, 2017: https://www.inchem.org/pages/pims.html
In patients with normal renal function, a small amount of furosemide is metabolized in the liver to the defurfurylated derivative, 4-chloro-5-sulfamoylanthranilic acid. ...
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2831
The half-life from the dose of 40 mg furosemide was 4 hours following oral administration and 4.5 hours following intravenous administration. The terminal half-life of furosemide is approximately 2 hours following parenteral administration. The terminal half-life may be increased up to 24 hours in patients with severe renal failure.
To study the pharmacokinetics of furosemide (fursemide; Lasix) and its acyl glucuronide and to analyze the pharmacodynamic response, a study was conducted in 7 healthy subjects, mean age 34 yr, who received a single oral 80 mg dose of furosemide in tablet form. Two half-lives were distinguished in the plasma elimination of furosemide and its conjugate, with values of 1.25 and 30.4 hr for furosemide and 1.31 and 33.2 hr for the conjugate. ...
Vree TB et al; J Pharm Pharmacol (Nov): 964-9 (1995)
In dogs, ... the elimination half life /is/ approximately 1-1.5 hours.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 644
Various investigators have reported a wide range of elimination half-lives for furosemide. In one study, the elimination half-life averaged about 30 minutes in healthy patients who received 20-120 mg of the drug IV. In another study, the elimination half-life averaged 9.7 hours in patients with advanced renal failure who received 1 g of furosemide IV. The elimination half-life was more prolonged in 1 patient with concomitant liver disease.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2831
The serum half-life in therapeutic doses is 92 minutes; increasing in patients with uremia; congestive heart failure and cirrhosis as well as in the neonate and aged patients. In such patients the half-life may be extended to 20 hours.
International Programme on Chemical Safety (IPCS); Poisons Information Monograph (PIM) No. 240, Furosemide (July 1997). Available from, as of April 18, 2017: https://www.inchem.org/pages/pims.html
Furosemide promotes diuresis by blocking tubular reabsorption of sodium and chloride in the proximal and distal tubules, as well as in the thick ascending loop of Henle. This diuretic effect is achieved through the competitive inhibition of sodium-potassium-chloride cotransporters (NKCC2) expressed along these tubules in the nephron, preventing the transport of sodium ions from the lumenal side into the basolateral side for reabsorption. This inhibition results in increased excretion of water along with sodium, chloride, magnesium, calcium, hydrogen, and potassium ions. As with other loop diuretics, furosemide decreases the excretion of uric acid. Furosemide exerts direct vasodilatory effects, which results in its therapeutic effectiveness in the treatment of acute pulmonary edema. Vasodilation leads to reduced responsiveness to vasoconstrictors, such as angiotensin II and noradrenaline, and decreased production of endogenous natriuretic hormones with vasoconstricting properties. It also leads to increased production of prostaglandins with vasodilating properties. Furosemide may also open potassium channels in resistance arteries. The main mechanism of action of furosemide is independent of its inhibitory effect on carbonic anhydrase and aldosterone.
Though both in vivo and in vitro studies have demonstrated an anticonvulsant effect of the loop diuretic furosemide, the precise mechanism behind this effect is still debated. The current study investigates the effect of furosemide on Cs-induced epileptiform activity (Cs-FP) evoked in area CA1 of rat hippocampal slices in the presence of Cs(+) (5mM) and ionotropic glutamatergic and GABAergic receptor antagonists. As this model diverges in several respects from other epilepsy models it can offer new insight into the mechanism behind the anticonvulsive effect of furosemide. The present study shows that furosemide suppresses the Cs-FP in a dose-dependent manner with a near complete block at concentrations = 1.25 mM. Because furosemide targets several types of ion transporters we examined the effect of more selective antagonists. Bumetanide (20 uM), which selectively inhibits the Na-K-2Cl co-transporter (NKCC1), had no significant effect on the Cs-FP. VU0240551 (10 uM), a selective antagonist of the K-Cl co-transporter (KCC2), reduced the ictal-like phase by 51.73 +/- 8.5% without affecting the interictal-like phase of the Cs-FP. DIDS (50 uM), a nonselective antagonist of Cl(-)/HCO3(-)-exchangers, Na(+)-HCO3(-)-cotransporters, chloride channels and KCC2, suppressed the ictal-like phase by 60.8 +/- 8.1% without affecting the interictal-like phase. At 500 uM, DIDS completely suppressed the Cs-FP. Based on these results we propose that the anticonvulsant action of furosemide in the Cs(+)-model is exerted through blockade of the neuronal KCC2 and Na(+)-independent Cl(-)/HCO3(-)-exchanger (AE3) leading to stabilization of the activity-induced intracellular acidification in CA1 pyramidal neurons.
PMID:26301821 Uwera J et al; Brain Res 1625: 1-8 (2015)
Sodium chloride reabsorption in the thick ascending limb of the loop of Henle is mediated by the Na(+)-K(+)-2Cl(-) cotransporter (NKCC2). The loop diuretic furosemide is a potent inhibitor of NKCC2. However, less is known about the mechanism regulating the electrolyte transporter. Considering the well-established effects of nitric oxide on NKCC2 activity, cGMP is likely involved in this regulation. cGMP-dependent protein kinase I (cGKI; PKGI) is a cGMP target protein that phosphorylates different substrates after activation through cGMP. We investigated the potential correlation between the cGMP/cGKI pathway and NKCC2 regulation. We treated wild-type (wt) and cGKIa-rescue mice with furosemide. cGKIa-rescue mice expressed cGKIa only under the control of the smooth muscle-specific transgelin (SM22) promoter in a cGKI deficient background. Furosemide treatment increased the urine excretion of sodium and chloride in cGKIa-rescue mice compared to that in wt mice. We analyzed the phosphorylation of NKCC2 by western blotting and immunostaining using the phosphospecific antibody R5. The administration of furosemide significantly increased the phosphorylated NKCC2 signal in wt but not in cGKIa-rescue mice. NKCC2 activation led to its phosphorylation and membrane translocation. To examine whether cGKI was involved in this process, we analyzed vasodilator-stimulated phosphoprotein, which is phosphorylated by cGKI. Furosemide injection resulted in increased vasodilator-stimulated phosphoprotein phosphorylation in wt mice. We hypothesize that furosemide administration activated cGKI, leading to NKCC2 phosphorylation and membrane translocation. This cGKI-mediated pathway could be a mechanism to compensate for the inhibitory effect of furosemide on NKCC2.
PMID:26183401 Limmer F et al; FEBS J 282 (19): 3786-98 (2015)

NDC Package Code : 82348-105
Start Marketing Date : 1995-12-18
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-11-20
Pay. Date : 2013-11-05
DMF Number : 2565
Submission : 1975-11-12
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2649
Submission : 1976-04-02
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1834
Submission : 1971-11-22
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5993
Submission : 1985-08-26
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
17 Mar 2025
Reply
24 Jan 2025
Reply
21 Jan 2025
Reply
21 Sep 2024
Reply
12 Mar 2024
Reply
29 Jan 2024
Reply
01 Dec 2023
Reply
13 Jul 2023
Reply
13 Apr 2023
Reply
15 Feb 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
33
PharmaCompass offers a list of Furosemide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Furosemide manufacturer or Furosemide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Furosemide manufacturer or Furosemide supplier.
PharmaCompass also assists you with knowing the Furosemide API Price utilized in the formulation of products. Furosemide API Price is not always fixed or binding as the Furosemide Price is obtained through a variety of data sources. The Furosemide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Furosemide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Furosemide, including repackagers and relabelers. The FDA regulates Furosemide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Furosemide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Furosemide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Furosemide supplier is an individual or a company that provides Furosemide active pharmaceutical ingredient (API) or Furosemide finished formulations upon request. The Furosemide suppliers may include Furosemide API manufacturers, exporters, distributors and traders.
click here to find a list of Furosemide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Furosemide DMF (Drug Master File) is a document detailing the whole manufacturing process of Furosemide active pharmaceutical ingredient (API) in detail. Different forms of Furosemide DMFs exist exist since differing nations have different regulations, such as Furosemide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Furosemide DMF submitted to regulatory agencies in the US is known as a USDMF. Furosemide USDMF includes data on Furosemide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Furosemide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Furosemide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Furosemide Drug Master File in Japan (Furosemide JDMF) empowers Furosemide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Furosemide JDMF during the approval evaluation for pharmaceutical products. At the time of Furosemide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Furosemide suppliers with JDMF on PharmaCompass.
A Furosemide CEP of the European Pharmacopoeia monograph is often referred to as a Furosemide Certificate of Suitability (COS). The purpose of a Furosemide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Furosemide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Furosemide to their clients by showing that a Furosemide CEP has been issued for it. The manufacturer submits a Furosemide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Furosemide CEP holder for the record. Additionally, the data presented in the Furosemide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Furosemide DMF.
A Furosemide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Furosemide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Furosemide suppliers with CEP (COS) on PharmaCompass.
A Furosemide written confirmation (Furosemide WC) is an official document issued by a regulatory agency to a Furosemide manufacturer, verifying that the manufacturing facility of a Furosemide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Furosemide APIs or Furosemide finished pharmaceutical products to another nation, regulatory agencies frequently require a Furosemide WC (written confirmation) as part of the regulatory process.
click here to find a list of Furosemide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Furosemide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Furosemide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Furosemide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Furosemide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Furosemide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Furosemide suppliers with NDC on PharmaCompass.
Furosemide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Furosemide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Furosemide GMP manufacturer or Furosemide GMP API supplier for your needs.
A Furosemide CoA (Certificate of Analysis) is a formal document that attests to Furosemide's compliance with Furosemide specifications and serves as a tool for batch-level quality control.
Furosemide CoA mostly includes findings from lab analyses of a specific batch. For each Furosemide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Furosemide may be tested according to a variety of international standards, such as European Pharmacopoeia (Furosemide EP), Furosemide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Furosemide USP).
