
Synopsis
Synopsis
0
EU WC
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
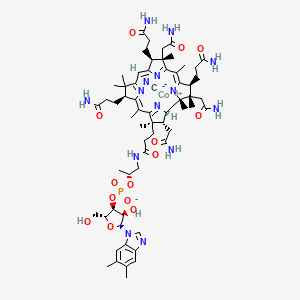

1. B 12, Vitamin
2. B12, Vitamin
3. Cobalamin
4. Cobalamins
5. Eritron
6. Vitamin B 12
7. Vitamin B12
1. Vitamin B12
2. 68-19-9
3. Cyanocob(iii)alamin
4. Vitamin B12 Complex
5. Dsstox_cid_24346
6. Dsstox_rid_80159
7. Dsstox_gsid_44346
8. Cobalamin (1+)
9. Mfcd00151092
10. Cas-68-19-9
11. Ncgc00016302-01
12. Prestwick_564
13. Vitamin B12 Nos
14. Vitamin B12 (charged)
15. Hms1569g08
16. Hms2096g08
17. Hms3713g08
18. Tox21_110358
19. Tox21_113664
20. Akos015894306
21. Akos037515760
22. Ccg-220433
23. Db00115
24. Ncgc00249888-01
25. 13408-78-1
26. Q55167869
27. Cobinamide,dihydrogenphosphate(ester),inner Salt,3'-ester With(5,6-dimethyl-1-a-d-ribofuranosyl-1h-benzimidazole-kn3),ion(1+)(9ci)
| Molecular Weight | 1355.4 g/mol |
|---|---|
| Molecular Formula | C63H88CoN14O14P |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 26 |
| Exact Mass | 1354.567399 g/mol |
| Monoisotopic Mass | 1354.567399 g/mol |
| Topological Polar Surface Area | 476 Ų |
| Heavy Atom Count | 93 |
| Formal Charge | 0 |
| Complexity | 3220 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 6 | |
|---|---|
| Drug Name | Cyanocobalamin |
| PubMed Health | Cyanocobalamin (Vitamin B-12) |
| Drug Classes | Diagnostic Agent, Vitamin B12 Absorption, Nutritive Agent |
| Drug Label | Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a mole... |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Luitpold |
| 2 of 6 | |
|---|---|
| Drug Name | Nascobal |
| PubMed Health | Cyanocobalamin (Vitamin B-12) |
| Drug Classes | Diagnostic Agent, Vitamin B12 Absorption, Nutritive Agent |
| Drug Label | Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a mole... |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.5mg/spray |
| Market Status | Prescription |
| Company | Par Pharm |
| 3 of 6 | |
|---|---|
| Drug Name | Vibisone |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 4 of 6 | |
|---|---|
| Drug Name | Cyanocobalamin |
| PubMed Health | Cyanocobalamin (Vitamin B-12) |
| Drug Classes | Diagnostic Agent, Vitamin B12 Absorption, Nutritive Agent |
| Drug Label | Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a mole... |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Luitpold |
| 5 of 6 | |
|---|---|
| Drug Name | Nascobal |
| PubMed Health | Cyanocobalamin (Vitamin B-12) |
| Drug Classes | Diagnostic Agent, Vitamin B12 Absorption, Nutritive Agent |
| Drug Label | Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a mole... |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Spray, metered |
| Route | Nasal |
| Strength | 0.5mg/spray |
| Market Status | Prescription |
| Company | Par Pharm |
| 6 of 6 | |
|---|---|
| Drug Name | Vibisone |
| Active Ingredient | Cyanocobalamin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
Hematinics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Vitamin B12 is used in the treatment of pernicious anemia and other vitamin B12 deficiency states. ... Cyanocobalamin ... usually indicated in patients with malabsorption of vitamin B12, such as those with tropical or nontropical sprue (idiopathic steatorrhea, gluten-induced enteropathy); partial or total gastrectomy; regional enteritis; gastroenterostomy; ileal resection; or malignancies, granulomas, strictures, or anastomoses involving the ileum. When the secretion of intrinsic factor is decreased by lesions that destroy the gastric mucosa (eg, following ingestion of corrosives or in patients with extensive GI neoplasia) or by gastric atrophy secondary to multiple sclerosis, certain endocrine disorders, or iron deficiency, or when antibodies to intrinsic factor are present in gastric juice, absorption of vitamin B12 is decreased and cyanocobalamin ... may be required. Malabsorption of vitamin B12 may also be caused by competition for vitamin B12 by bacteria (blind loop syndrome) or by fish tapeworm, Diphyllobothrium latum, or by admin of certain drugs.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3325
The individual with an uncomplicated pernicious anemia, in which the abnormality is restricted to a mild or moderate anemia ... will respond quite well to the admin of vitamin B12 alone.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1332
... Patients with neurological change or severe leukopenia or thrombocytopenia associated with infection or bleeding require emergency treatment. The older individual with a severe anemia (hematocrit less than 20%) is likely to have tissue hypoxia, cerebrovascular insufficiency, and congestive heart failure. Effective therapy must not wait for detailed diagnostic tests. ... The patient should receive intramuscular injections of 100 ug of cyanocobalamin and 1 to 5 mg of folic acid.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1332
For more Therapeutic Uses (Complete) data for CYANOCOBALAMIN (13 total), please visit the HSDB record page.
Cyanocobalamin injection is extremely safe when given by the intramuscular or deep subcutaneous route, but it should never be given intravenously.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1331
Cyanocobalamin should not be used in patients with early Leber's disease (hereditary optic nerve atrophy), since rapid optic nerve atrophy has been reported following admin of the drug to these patients. Vitamin B12 is contraindicated in patients who have experienced hypersensitivity reactions to the vitamin or to cobalt.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3326
/"SHOTGUN"/ ... VITAMIN THERAPY IN TREATMENT OF ... DEFICIENCY CAN BE DANGEROUS. ... THERE IS DANGER THAT SUFFICIENT FOLIC ACID WILL BE GIVEN TO RESULT IN HEMATOLOGICAL RECOVERY; HOWEVER, THIS MAY MASK CONTINUED VIT-B DEFICIENCY & NEUROLOGICAL DAMAGE WILL DEVELOP OR PROGRESS IF ALREADY PRESENT.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1332
Maternal Medication usually Compatible with Breast-Feeding: B12: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
Serum potassium concn should be monitored during early vitamin B12 therapy & potassium admin is necessary, since fatal hypokalemia could occur upon conversion of megaloblastic anemia to normal erythropoesis with vitamin B12 as a result of increased erythrocyte potassium requirements. Because vitamin B12 deficiency may suppress the signs of polycythemia vera, treatment with cyanocobalamin may unmask this condition. The increase in nucleic acid degradation produced by admin vitamin B12 to vitamin B12- deficient patients could result in gout in susceptible individuals. Therapeutic response to vitamin B12 may be impaired by concurrent infection, uremia, folic acid or iron deficiency, or by drugs having bone marrow suppressant effects. Folic acid should be admin with extreme caution to patients with undiagnosed anemia, since folic acid may obscure the diagnosis of pernicious anemia by alleviating hematologic manifestations of the disease while allowing neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Vitamin preparations containing folic acid should be avoided by patients with pernicious anemia because folic acid may actually potentiate neurologic complications of vitamin B12 deficiency. Conversely, doses of cyanocobalamin exceeding 10 micrograms daily may improve folate-deficient megaloblastic anemia and obscure the true diagnosis.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3326
**Nasal spray** The cyanocobalamin nasal spray is indicated for the maintenance of vitamin B12 concentrations after normalization with intramuscular vitamin B12 therapy in patients with deficiency of this vitamin who have no nervous system involvement. Note: CaloMist, the nasal spray form, has not been evaluated for the treatment of newly diagnosed vitamin B12 deficiency. **Injection forms (subcutaneous, intramuscular)** These forms are indicated for vitamin B12 deficiencies due to various causes, with or without neurologic manifestations. Vitamin B12 deficiency is frequently caused by malabsorption, which is often associated with the following conditions: Addisonian (pernicious) anemia Gastrointestinal pathology, dysfunction, or surgery, including gluten enteropathy or sprue, small bowel bacterial overgrowth, total or partial gastrectomy Fish tapeworm infestation Malignancy of the pancreas or bowel Folic acid deficiency **Oral forms** Vitamin B12 supplements are widely available and indicated in patients who require supplementation for various reasons. Dose requirements for vitamin B12 which are higher than normal (caused by pregnancy, thyrotoxicosis, hemolytic anemia, hemorrhage, malignancy, hepatic and renal disease) can usually be achieved with oral supplementation. Oral products of vitamin B12 are not recommended in patients with malabsorption, as these forms are primarily absorbed in the gastrointestinal tract.
FDA Label
**General effects** Cyanocobalamin corrects vitamin B12 deficiency and improves the symptoms and laboratory abnormalities associated with pernicious anemia (megaloblastic indices, gastrointestinal lesions, and neurologic damage). This drug aids in growth, cell reproduction, hematopoiesis, nucleoprotein, and myelin synthesis. It also plays an important role in fat metabolism, carbohydrate metabolism, as well as protein synthesis. Cells that undergo rapid division (for example, epithelial cells, bone marrow, and myeloid cells) have a high demand for vitamin B12. **Parenteral cyanocobalamin effects** The parenteral administration of vitamin B12 rapidly and completely reverses the megaloblastic anemia and gastrointestinal symptoms of vitamin B12 deficiency. Rapid parenteral administration of vitamin B12 in deficiency related neurological damage prevents the progression of this condition. **Nasal spray effects** In 24 vitamin B12 deficient patients who were already stabilized on intramuscular (IM) vitamin B12 therapy, single daily doses of intranasal cyanocobalamin for 8 weeks lead to serum vitamin B12 concentrations that were within the target therapeutic range (>200 ng/L).
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
B - Blood and blood forming organs
B03 - Antianemic preparations
B03B - Vitamin b12 and folic acid
B03BA - Vitamin b12 (cyanocobalamin and analogues)
B03BA01 - Cyanocobalamin
Absorption
Vitamin B12 is quickly absorbed from intramuscular (IM) and subcutaneous (SC) sites of injection; with peak plasma concentrations achieved about 1 hour after IM injection. Orally administered vitamin B12 binds to intrinsic factor (IF) during its transport through the stomach. The separation of Vitamin B12 and IF occurs in the terminal ileum when calcium is present, and vitamin B12 is then absorbed into the gastrointestinal mucosal cells. It is then transported by transcobalamin binding proteins. Passive diffusion through the intestinal wall can occur, however, high doses of vitamin B12 are required in this case (i.e. >1 mg). After the administration of oral doses less than 3 mcg, peak plasma concentrations are not reached for 8 to 12 hours, because the vitamin is temporarily retained in the wall of the lower ileum.
Route of Elimination
This drug is partially excreted in the urine. According to a clinical study, approximately 3-8 mcg of vitamin B12 is secreted into the gastrointestinal tract daily via the bile. In patients with adequate levels of intrinsic factor, all except approximately 1 mcg is reabsorbed. When vitamin B12 is administered in higher doses that saturate the binding capacity of plasma proteins and the liver, the unbound vitamin B12 is eliminated rapidly in the urine. The body storage of vitamin B12 is dose-dependent.
Volume of Distribution
Cobalamin is distributed to tissues and stored mainly in the liver and bone marrow.
Clearance
During vitamin loading, the kidney accumulates large amounts of unbound vitamin B12. This drug is cleared partially by the kidney, however, multiligand receptor _megalin_ promotes the reuptake and reabsorption of vitamin B12 into the body,.
IN MICE INJECTED IV WITH VITAMIN B12, THE VITAMIN ACCUMULATED RAPIDLY IN THE PLACENTA & WAS TRANSFERRED SLOWLY TO THE FETUSES. PEAK CONCN IN THE FETUSES WAS REACHED 24 HR AFTER DOSING, & FETAL ACCUMULATION WAS DOSE-DEPENDENT.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 636
IN /MICE/ VITAMIN B12 PRESENTS UNUSUAL PATTERN OF PLACENTAL TRANSFER, FOR EVEN WITH 0.20 UG MATERNAL DOSE AVG FETAL CONCN IS 130 TIMES HIGHER THAN MATERNAL ONE. THIS INDICATES STRONGLY OPERATION OF SPECIFIC TRANSPORT MECHANISM FOR VITAMIN B12, POSSIBLY SIMILAR TO ITS GI ABSORPTION ...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 636
IN RATS, PLACENTAL TRANSFER OF VITAMIN B12 WAS SHOWN TO INCR DURING GESTATION. ALTHOUGH QUANTITY TRANSPORTED EACH DAY WAS PROPORTIONAL TO FETAL WT, THE AMT TRANSPORTED PER G OF PLACENTA INCR TEN-FOLD FROM DAY 10 TO DAY 19.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 636
Vitamin B12 is irregularly absorbed from the distal small intestine following oral administration. Dietary vitamin B12 is protein bound and this bond must be split by proteolysis and gastric acid before absorption. In the stomach, free vitamin B12 is attached to intrinsic factor; intrinsic factor a glycoprotein secreted by the gastric mucosa, is necessary for active absorption of the vitamin from the GI tract. The vitamin B12-intrinsic factor complex passes into the intestine, where much of the complex is transiently retained at specific receptor sites in the wall of the lower ileum before the vitamin B12 portion is absorbed into systemic circulation.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3325
For more Absorption, Distribution and Excretion (Complete) data for CYANOCOBALAMIN (9 total), please visit the HSDB record page.
Vitamin B12 or cyanocobalamin obtained from food is initially bound by _haptocorrin_, a protein found in the saliva with high affinity for B12. This forms a _haptocorrin-B12_ complex. Cyanocobalamin passes through the stomach and is protected from acid degradation due to its binding to haptocorrin. In the duodenum, pancreatic _proteases_ release cobalamin from the _haptocorrin-B12 complex_ and from other proteins containing protein-bound B12 that have been ingested. Following this, the binding of cobalamin to a second glycoprotein, _intrinsic factor_, promotes its uptake by terminal ileum mucosal cells by a process called _cubilin_/AMN receptor-mediated endocytosis. After absorption into enterocytes, intrinsic factor is broken down in the lysosome, and cobalamin is then released into the bloodstream. The transporter ABCC1, found in the basolateral membrane of intestinal epithelial and other cells, exports cobalamin bound to transcobalamin out of the cell. Cyanocobalamin then passes through the portal vein in the liver, and then reaches the systemic circulation. The active forms of cyanocobalamin are _methylcobalamin_ and _adenosylcobalamin_,.
Vitamin B12 is believed to be converted to coenzyme form in the liver and is probably stored in tissues in this form.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3325
Intracellular vitamin B12 is maintained as two active coenzymes methylcobalamin and deoxyadenasylcobalamin.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1328
Approximately 6 days (400 days in the liver).
HALF-LIFE OF IV ADMIN CYANOCOBALAMIN IN SERUM IS ABOUT 6 DAYS.
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 450
Vitamin B12 serves as a cofactor for _methionine synthase_ and _L-methylmalonyl-CoA mutase_ enzymes. Methionine synthase is essential for the synthesis of purines and pyrimidines that form DNA. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to _succinyl-CoA_ in the degradation of propionate, an important reaction required for both fat and protein metabolism. It is a lack of vitamin B12 cofactor in the above reaction and the resulting accumulation of methylmalonyl CoA that is believed to be responsible for the neurological manifestations of B12 deficiency. Succinyl-CoA is also necessary for the synthesis of hemoglobin. In tissues, vitamin B12 is required for the synthesis of _methionine_ from homocysteine. Methionine is required for the formation of S-adenosylmethionine, a methyl donor for nearly 100 substrates, comprised of DNA, RNA, hormones, proteins, as well as lipids. Without vitamin B12, tetrahydrofolate cannot be regenerated from 5-methyltetrahydrofolate, and this can lead to functional folate deficiency,. This reaction is dependent on methylcobalamin (vitamin B12) as a co-factor and is also dependent on folate, in which the methyl group of methyltetrahydrofolate is transferred to homocysteine to form _methionine_ and _tetrahydrofolate_. Vitamin B12 incorporates into circulating folic acid into growing red blood cells; retaining the folate in these cells. A deficiency of vitamin B12 and the interruption of this reaction leads to the development of megaloblastic anemia.
CYANOCOBALAMIN STIMULATES RETICULOCYTES, THUS PLAYING IMPORTANT ROLE IN HEMATOPOIESIS IN THAT, TOGETHER WITH FOLIC ACID, IT IS INVOLVED IN FORMATION OF DEOXYRIBONUCLEOTIDES FROM RIBONUCLEOTIDES.
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 450
Vitamin B12 is converted to Coenzyme B12 in tissues, and as such is essential for conversion of methylmalonate to succinate and synthesis of methionine from homocysteine, a reaction which also requires folate. ... Vitamin B12 also may be involved in maintaining sulfhydryl (SH) groups in the reduced form required by many SH-activated enzyme systems. Through these reactions, vitamin B12 is associated with fat and carbohydrate metabolism and protein synthesis.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3325

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Vitamin B12 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vitamin B12 manufacturer or Vitamin B12 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vitamin B12 manufacturer or Vitamin B12 supplier.
PharmaCompass also assists you with knowing the Vitamin B12 API Price utilized in the formulation of products. Vitamin B12 API Price is not always fixed or binding as the Vitamin B12 Price is obtained through a variety of data sources. The Vitamin B12 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DODEX manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DODEX, including repackagers and relabelers. The FDA regulates DODEX manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DODEX API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of DODEX manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A DODEX supplier is an individual or a company that provides DODEX active pharmaceutical ingredient (API) or DODEX finished formulations upon request. The DODEX suppliers may include DODEX API manufacturers, exporters, distributors and traders.
click here to find a list of DODEX suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A DODEX DMF (Drug Master File) is a document detailing the whole manufacturing process of DODEX active pharmaceutical ingredient (API) in detail. Different forms of DODEX DMFs exist exist since differing nations have different regulations, such as DODEX USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A DODEX DMF submitted to regulatory agencies in the US is known as a USDMF. DODEX USDMF includes data on DODEX's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The DODEX USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of DODEX suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The DODEX Drug Master File in Japan (DODEX JDMF) empowers DODEX API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the DODEX JDMF during the approval evaluation for pharmaceutical products. At the time of DODEX JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of DODEX suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a DODEX Drug Master File in Korea (DODEX KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of DODEX. The MFDS reviews the DODEX KDMF as part of the drug registration process and uses the information provided in the DODEX KDMF to evaluate the safety and efficacy of the drug.
After submitting a DODEX KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their DODEX API can apply through the Korea Drug Master File (KDMF).
click here to find a list of DODEX suppliers with KDMF on PharmaCompass.
A DODEX CEP of the European Pharmacopoeia monograph is often referred to as a DODEX Certificate of Suitability (COS). The purpose of a DODEX CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of DODEX EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of DODEX to their clients by showing that a DODEX CEP has been issued for it. The manufacturer submits a DODEX CEP (COS) as part of the market authorization procedure, and it takes on the role of a DODEX CEP holder for the record. Additionally, the data presented in the DODEX CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the DODEX DMF.
A DODEX CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. DODEX CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of DODEX suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing DODEX as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for DODEX API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture DODEX as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain DODEX and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a DODEX NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of DODEX suppliers with NDC on PharmaCompass.
DODEX Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DODEX GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DODEX GMP manufacturer or DODEX GMP API supplier for your needs.
A DODEX CoA (Certificate of Analysis) is a formal document that attests to DODEX's compliance with DODEX specifications and serves as a tool for batch-level quality control.
DODEX CoA mostly includes findings from lab analyses of a specific batch. For each DODEX CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DODEX may be tested according to a variety of international standards, such as European Pharmacopoeia (DODEX EP), DODEX JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DODEX USP).

