
Synopsis
Synopsis
0
JDMF
0
VMF
0
Canada
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
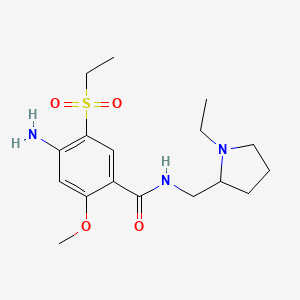

1. 4-amino-n-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-methoxybenzamide
2. Barnetil
3. Dan 2163
4. Dan-2163
5. Lin 1418
6. Lin-1418
7. N-(ethyl-1-pyrrolidinyl- 2-methyl)methoxy-2-ethylsulfonyl-5-benzamide
8. Solian
9. Sultopride
10. Sultopride Hydrochloride
1. 71675-85-9
2. Solian
3. Aminosultopride
4. Deniban
5. Amisulprida
6. Dan 2163
7. Amisulpridum
8. Socian
9. Amisulpride [inn]
10. Barhemsys
11. Dan-2163
12. 4-amino-n-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide
13. 4-amino-n-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamide
14. Apd421
15. Sulamid
16. C17h27n3o4s
17. Amisulpride (inn)
18. 4-amino-n-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-methoxybenzamide
19. Amisulpride Free Base
20. 4-amino-n-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-o-anisamide
21. Nsc-760085
22. 4-amino-n-((1-ethylpyrrolidin-2-yl)methyl)-5-(ethylsulfonyl)-2-methoxybenzamide
23. 4-amino-n-[(1-ethylpyrrolidin-2-yl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide
24. Amisulpiride
25. Chebi:64045
26. Apd-421
27. 71675-85-9 (free Base)
28. 8110r61i4u
29. Ncgc00092310-03
30. Benzamide, 4-amino-n-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-methoxy-
31. Dsstox_cid_22613
32. Dsstox_rid_80058
33. Dsstox_gsid_42613
34. Amisulpridum [inn-latin]
35. Amisulprida [inn-spanish]
36. Sulpitac
37. 4-amino-n-[(1-ethyl-2-pyrrolidinyl)methyl]-5-ethylsulfonyl-2-methoxybenzamide
38. Smr000449309
39. Deniban (tn)
40. Cas-71675-85-9
41. Solian (tn)
42. Amisulpride [inn:ban]
43. Einecs 275-831-7
44. Unii-8110r61i4u
45. Barhemsys (tn)
46. 4-amino-n-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-anisamid
47. Amisulpride- Bio-x
48. (plusmn)-amisulpride
49. Mfcd00866691
50. Amisulpride [mi]
51. Sl-91.1077
52. Amisulpride [mart.]
53. Schembl34126
54. (+/-)-amisulpride
55. Amisulpride [who-dd]
56. Gtpl963
57. Mls000758258
58. Mls000759450
59. Mls001424039
60. Mls006011769
61. Chembl243712
62. Dtxsid5042613
63. Amisulpride, >=98% (hplc)
64. Bdbm81790
65. Amisulpride [orange Book]
66. Amisulpride For System Suitability
67. Amisulpride [ep Monograph]
68. Bcpp000404
69. Hms2051k10
70. Hms2235m16
71. Hms3263d07
72. Hms3268l09
73. Hms3369k09
74. Hms3393k10
75. Hms3413g07
76. Hms3657e11
77. Hms3677g07
78. Hms3713n06
79. Hms3884a09
80. Pharmakon1600-01502285
81. Bcp02089
82. Nsc_2159
83. Tox21_113392
84. Tox21_501133
85. Nsc760085
86. Stk635172
87. Stl454297
88. Akos005567252
89. Tox21_113392_1
90. Ac-6820
91. Bcp9000294
92. Ccg-100860
93. Ccg-220622
94. Ccg-222437
95. Cs-1791
96. Db06288
97. Lp01133
98. Nc00110
99. Nsc 760085
100. Sdccgsbi-0633795.p001
101. 4-amino-5-(ethanesulfonyl)-n-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide
102. Ncgc00092310-01
103. Ncgc00092310-02
104. Ncgc00092310-04
105. Ncgc00092310-06
106. Ncgc00092310-16
107. Ncgc00261818-01
108. As-13890
109. Ba164158
110. Hy-14545
111. Amisulpride 100 Microg/ml In Acetonitrile
112. Cas_71675-85-9
113. Ft-0630805
114. S1280
115. Sw197490-3
116. D07310
117. Ab00639933-07
118. Ab00639933-09
119. Ab00639933_10
120. 675a859
121. A837282
122. L000106
123. Q418785
124. Sr-01000759303
125. Sr-01000759303-5
126. W-104511
127. Brd-a60197193-001-05-4
128. Amisulpride, British Pharmacopoeia (bp) Reference Standard
129. Amisulpride, European Pharmacopoeia (ep) Reference Standard
130. 4-amino-n-[(1-ethyl-2-pyrrolidinyl) Methyl]-5-(ethylsulfonyl)-2-benzamide
131. 4-amino-n-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-benzamide
132. 4-amino-5-ethanesulfonyl-n-(1-ethyl-pyrrolidin-2-ylmethyl)-2-methoxy-benzamide
133. 4-amino-n-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulf Onyl)-2-benzamide Dan 2163
134. 4-amino-n-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)- 2-methoxybenzamide
135. 4-azanyl-n-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxy-benzamide
136. Amisulpride For System Suitability, European Pharmacopoeia (ep) Reference Standard
137. Benzamide,4-amino-n-[[(2s)-1-ethyl-2-pyrrolidinyl]methyl]-5-(ethylsulfonyl)-2-methoxy-
138. 4-amino-n-[(1-ethylpyrrolidin-2-yl)methyl]-5-(ethylsulfonyl)-2-methoxybenzenecarboximidic Acid
| Molecular Weight | 369.5 g/mol |
|---|---|
| Molecular Formula | C17H27N3O4S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 369.17222752 g/mol |
| Monoisotopic Mass | 369.17222752 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 549 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Intravenous amisulpride is indicated in adults for the prevention of postoperative nausea and vomiting, either alone or in combination with an antiemetic of a different class. It is also indicated for the treatment of postoperative nausea and vomiting in patients who have received anti-emetic prophylaxis with an agent of a different class or have not received prophylaxis. Oral amisulpride is indicated for the treatment of acute and chronic schizophrenic disorders, characterized by positive symptoms with delusions, hallucinations, thought disorders, hostility and suspicious behavior; or primarily negative symptoms (deficit syndrome) with blunted affect, emotional and social withdrawal. Amisulpride also controls secondary negative symptoms in productive conditions as well as affective disorders such as depressive mood or retardation.
Amisulpride is a selective dopamine D2 and D3 receptor antagonist with no affinity towards other dopamine receptor subtypes. Amisulpride is an atypical antipsychotic agent that works as an antagonist at dopamine receptors in the limbic system. Since it works preferentially in the limbic system, amisulpride is less likely to be associated with extrapyramidal adverse effects than other atypical antipsychotic agents. Amisulpride has no affinity for serotonin, alpha-adrenergic, H1-histamine, cholinergic, and sigma receptors. In clinical trials, amisulpride improved reduced secondary negative symptoms, affective symptoms, and psychomotor retardation in patients with acute exacerbation of schizophrenia. Notably, amisulpride has a differential target binding profile at different doses: at low doses, amisulpride selectively binds to presynaptic dopamine autoreceptors. At high doses, it preferentially binds to post-synaptic dopamine receptors. This explains how amisulpride reduces negative symptoms at low doses and mediates antipsychotic effects at high doses. One study alluded that the antinociceptive effects of amisulpride are mediated through opioid receptor acvitation and D2 receptor antagonism. The actions of amisulpride at opioid receptors may explain its pro-convulsant properties. Amisulpride is also an antiemetic agent that prevents and alleviates postoperative nausea and vomiting. It primarily works by blocking dopamine signalling in the chemoreceptor trigger zone, which is a brain area that relays stimuli to the vomiting center. In clinical trials comprising Caucasian and Japanese subjects, amisulpride caused dose- and concentration-dependent prolongation of the QT interval; thus, intravenous infusion under a strict dosing regimen and close monitoring of patients with pre-existing cardiovascular conditions are recommended. Amisulpride increases plasma prolactin levels, leading to an association with benign pituitary tumours such as prolactinoma.
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N05AL05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AL - Benzamides
N05AL05 - Amisulpride
Absorption
Following oral administration, amisulpride is rapidly absorbed with absolute bioavailability of 48%. Amisulpride has two absorption peaks, with one rapidly achieved within one hour post-dose and a second peak occurring between three to four hours post-dose. Following oral administration of a 50 mg dose, two peak plasma concentrations were 39 3 and 54 4 ng/mL. Following intravenous administration, the peak plasma concentration of amisulpride is achieved at the end of the infusion period and the plasma concentration decreases by 50% within approximately 15 minutes. The AUC(0-) increases dose-proportionally in the dose range from 5 mg to 40 mg, which is about four times the maximum recommended dose. In healthy patients receiving intravenous amisulpride, the mean (SD) Cmax was 200 (139) ng/mL at the dose of 5 mg and 451 (230) ng/mL at the dose of 10 mg. The AUC ranged from 136 to 154 ng x h/mL in the dose range of 5 mg to 10 mg. In patients undergoing surgery, the mean (SD) Cmax ranged from 127 (62) to 161 (58) ng/mL at the dose of 5 mg. At the dose of 10 mg, it was 285 (446) ng/mL. The AUC ranged from 204 to 401 ng x h/mL.
Route of Elimination
Following intravenous administration, about 74% of amisulpride is excreted in urine, where 58% of the recovered dose was excreted as unchanged amisulpride. About 23% of the dose is excreted in feces, with 20% of the excreted dose as unchanged parent drug. Following intravenous administration, about four metabolites were identified in urine and feces, accounting for less than 7% of the total dose administered. About 22 to 25% of orally administered amisulpride is excreted in urine, mostly as the unchanged parent drug.
Volume of Distribution
Following oral administration, the volume of distribution is 5.8 L/kg. Following intravenous infusion, the mean volume of distribution of amisulpride is estimated to be 127 to 144 L in surgical patients and 171 L in healthy subjects.
Clearance
The plasma clearance of amisulpride is 20.6 L/h in surgical patients and 24.1 L/h in healthy subjects following intravenous administration. Renal clearance was estimated to be 20.5 L/hr (342 mL/min) in healthy subjects.
Amisulpride undergoes minimal metabolism and its metabolites in plasma are largely undetectable. Two identified metabolites, formed by de-ethylation and oxidation, are pharmacologically inactive and account for approximately 4% of the dose. Metabolites remain largely uncharacterized. Metabolism of amisulpride does not involve cytochrome P450 enzymes.
Elimination is biphasic. The elimination half-life of amisulpride is approximately 12 hours after an oral dose. The mean elimination half-life is approximately four to five hours in both healthy subjects and patients undergoing surgery receiving intravenous amisulpride.
Dopamine is an essential and critical neurotransmitter produced in the substantia nigra and ventral tegmental regions of the brain. Dopaminergic projection function in the nigrostriatal, mesolimbic, and mesocortical systems. Hyperactive dopamine transmission in the mesolimbic areas, or dopamine dysregulation in various major brain regions, is understood as the key driver of positive and negative symptoms of schizophrenia. Many antipsychotic agents act as D2 receptor antagonists, as with amisulpride. Amisulpride is a selective dopamine D2 and D3 receptor antagonist. It has high preferential activity towards dopamine receptors in the limbic system rather than the striatum, leading to a lower risk of extrapyramidal side effects than other atypical antipsychotic agents. At low doses, amisulpride reduces negative symptoms of schizophrenia by blocking pre-synaptic dopamine D2 and D3 receptors, increasing the levels of dopamine in the synaptic cleft and facilitating dopaminergic transmission. At higher doses, amisulpride blocks postsynaptic receptors, inhibiting dopaminergic hyperactivity: this explains the drug improving positive symptoms. Amisulpride also works as an antagonist at 5-HT7A receptors and 5-HT2A receptors, which may be related to its antidepressant effects. The chemoreceptor trigger zone (CTZ), also commonly known as the area postrema (AP), is an important brain region located within the dorsal surface of the medulla oblongata. CTZ is involved in emesis: it contains receptors, such as dopamine receptors, that are activated in response to emetic agents in the blood and relay information to the vomiting center, which is responsible for inducing the vomiting reflex. Amisulpride is an antiemetic agent that works to limit signals that promote nausea and vomiting. Amisulpride binds to D2 and D3 receptors in the CTZ, leading to reduced dopaminergic signalling into the vomiting center.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
83
PharmaCompass offers a list of Amisulpride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amisulpride manufacturer or Amisulpride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amisulpride manufacturer or Amisulpride supplier.
PharmaCompass also assists you with knowing the Amisulpride API Price utilized in the formulation of products. Amisulpride API Price is not always fixed or binding as the Amisulpride Price is obtained through a variety of data sources. The Amisulpride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Deniban manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Deniban, including repackagers and relabelers. The FDA regulates Deniban manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Deniban API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Deniban manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Deniban supplier is an individual or a company that provides Deniban active pharmaceutical ingredient (API) or Deniban finished formulations upon request. The Deniban suppliers may include Deniban API manufacturers, exporters, distributors and traders.
click here to find a list of Deniban suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Deniban DMF (Drug Master File) is a document detailing the whole manufacturing process of Deniban active pharmaceutical ingredient (API) in detail. Different forms of Deniban DMFs exist exist since differing nations have different regulations, such as Deniban USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Deniban DMF submitted to regulatory agencies in the US is known as a USDMF. Deniban USDMF includes data on Deniban's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Deniban USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Deniban suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Deniban Drug Master File in Korea (Deniban KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Deniban. The MFDS reviews the Deniban KDMF as part of the drug registration process and uses the information provided in the Deniban KDMF to evaluate the safety and efficacy of the drug.
After submitting a Deniban KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Deniban API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Deniban suppliers with KDMF on PharmaCompass.
A Deniban CEP of the European Pharmacopoeia monograph is often referred to as a Deniban Certificate of Suitability (COS). The purpose of a Deniban CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Deniban EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Deniban to their clients by showing that a Deniban CEP has been issued for it. The manufacturer submits a Deniban CEP (COS) as part of the market authorization procedure, and it takes on the role of a Deniban CEP holder for the record. Additionally, the data presented in the Deniban CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Deniban DMF.
A Deniban CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Deniban CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Deniban suppliers with CEP (COS) on PharmaCompass.
A Deniban written confirmation (Deniban WC) is an official document issued by a regulatory agency to a Deniban manufacturer, verifying that the manufacturing facility of a Deniban active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Deniban APIs or Deniban finished pharmaceutical products to another nation, regulatory agencies frequently require a Deniban WC (written confirmation) as part of the regulatory process.
click here to find a list of Deniban suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Deniban as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Deniban API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Deniban as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Deniban and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Deniban NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Deniban suppliers with NDC on PharmaCompass.
Deniban Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Deniban GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Deniban GMP manufacturer or Deniban GMP API supplier for your needs.
A Deniban CoA (Certificate of Analysis) is a formal document that attests to Deniban's compliance with Deniban specifications and serves as a tool for batch-level quality control.
Deniban CoA mostly includes findings from lab analyses of a specific batch. For each Deniban CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Deniban may be tested according to a variety of international standards, such as European Pharmacopoeia (Deniban EP), Deniban JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Deniban USP).

