
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
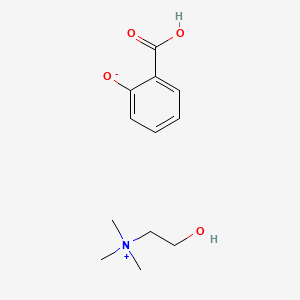

1. Mundasal
2. Mundisal
1. 2016-36-6
2. Syrap
3. (2-hydroxyethyl)trimethylammonium Salicylate
4. Choline Salicylic Acid Salt
5. Salicylic Acid Choline Salt
6. Choline, Salicylate (salt)
7. Cholin Salicylate
8. Salicylic Acid, Ion(1-), Choline
9. Choline Subsalicylate
10. 2-hydroxy-n,n,n-trimethylethanaminium 2-hydroxybenzoate
11. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, Salt With 2-hydroxybenzoic Acid (1:1)
12. Kd510k1iqw
13. Arthropan
14. 2-hydroxy-n,n,n-trimethylethanaminium Salt With 2-hydroxybenzoic Acid (1:1)
15. Actasal
16. Artrobione
17. Mundisal
18. Salicol
19. Satibon
20. Arret
21. 2-hydroxybenzoate;2-hydroxyethyl(trimethyl)azanium
22. 2-carboxyphenolate;2-hydroxyethyl(trimethyl)azanium
23. Choline Salicylate B
24. Cholinesalicylate
25. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, 2-hydroxybenzoate (1:1)
26. Cholini Salicylas
27. Salicilato De Colina
28. Cholini Salicylas [inn-latin]
29. Salicylate De Choline
30. Salicilato De Colina [inn-spanish]
31. Salicylate De Choline [inn-french]
32. Einecs 217-948-8
33. Unii-kd510k1iqw
34. Choline Salicylate [usan:inn:ban:jan]
35. Arthropan (tn)
36. Choline Salicylate (salt)
37. Schembl3960
38. Chebi:3668
39. Choline Salicylate [mi]
40. Chembl2104095
41. Choline Salicylate [inn]
42. Choline Salicylate [jan]
43. Dtxsid8062103
44. Choline Salicylate [usan]
45. Choline Salicylate [vandf]
46. Choline Salicylate [mart.]
47. Choline Salicylate [who-dd]
48. Choline Salicylate (jan/usan/inn)
49. Mfcd00242760
50. Akos030228339
51. Db14006
52. As-66082
53. Cs-0449641
54. D00810
55. (2-hydroxyethyl)trimethylazanium 2-hydroxybenzoate
56. A909239
57. (2-hydroxyethyl)trimethyl Ammonium Salicylate
58. 2-carboxyphenolate,2-hydroxyethyl(trimethyl)azanium
59. Q4499058
60. (2-hydroxyethyl)trimethylammonium 2-hydroxybenzoate
61. 2-hydroxy-n,n,n-trimethylethan-1-aminium 2-hydroxybenzoate
62. 2-hydroxy-n,n,n-trimethylethan-1-aminium 2-carboxyphenolate
63. Benzoic Acid, 2-hydroxy-, Ion(1-), 2-hydroxy-n,n,n-trimethylethanaminium
| Molecular Weight | 241.28 g/mol |
|---|---|
| Molecular Formula | C12H19NO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 241.13140809 g/mol |
| Monoisotopic Mass | 241.13140809 g/mol |
| Topological Polar Surface Area | 80.6 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 179 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
The oral gel is indicated for the relief of pain and discomfort of common mouth ulcers, cold sores, denture sore spots, infant teething and mouth ulcers, and sore spots due to orthodontic devices in children.
This is an anti-inflammatory and antipyretic medication,. If is often used in oral gel form for the relief of pain, discomfort, and inflammation caused by common mouth ulcers, cold sores, denture and sore spots, as well as mouth ulcers, and sore spots because of orthodontic devices.
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA03 - Choline salicylate
Absorption
Onset: 1-2 hr after ingestion In the oral form, choline salicylate is absorbed across the buccal mucosa. There is a need for caution not to exceed the stated dose and monitor for any signs of suggested salicylism, especially when this drug is used for infants. In one study, it was found that this drug was more rapidly absorbed than ASA (absorption t1/2 = 0.1 vs 0.36 h).
Route of Elimination
Both metabolites of choline salicylate, and a small amount of intact salicylic acid are excreted, primarily in the urine.
Volume of Distribution
0.15 L/kg (salicylate), and widely distributed throughout extracellular water and most tissues
The metabolism of salicylic acid is by glycine and phenolic or acyl glucuronate conjugation with small amounts of the drug undergoing hydroxylation. Extensively metabolized in the liver; inactive metabolites are excreted by the kidneys.
The plasma half-life of salicylic acid is 2-4 hours. Up to 15 30 h with larger doses due to saturation of liver metabolism capacity.
Choline salicylate relieves pain by inhibition of prostaglandin synthesis and reduces fever by acting on the hypothalamus heat-regulating center. It also inhibits the generation of impulses through the inhibition of cyclooxygenase enzyme (COX),. Cyclooxygenase is involved in the production of prostaglandins, in response to injury and after various other stimuli. The prostaglandins promote pain, swelling, and inflammation. The choline salicylate decreases inflammation and pain by reducing the production of these prostaglandins in the area of the mouth it is applied to.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7329
Submission : 1988-02-12
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Pharm-Rx has earned an outstanding reputation since its establishment in 1991, serving as a reputable importer and distributor of active ingredients to the pharmaceutical, nutritio...
About the Company : AROCHEM INDUSTRIES is a firm incepted in the year 1978 and is engaged in the business of manufacture and sell specialty organic chemicals, bulk drugs and other organic intermediate...

About the Company : ENOMARK Group of Companies stands as a prominent Contract Manufacturer, boasting a cutting-edge manufacturing facility in Ahmadabad (Gujarat) that complies with WHO-GMP standards. ...

About the Company : Kreative Organics Private Limited is a manufacturer of Active Pharmaceutical Ingredients (APIs) for the world market. Kreative started operations in 1990. Kreative was established ...

About the Company : S.S. PHARMACHEM is a professionally managed Bulk Drug & Chemical manufacturing unit headed by MR. SAGAR S. SANGEKAR & MR. SANJAY S. SANGEKAR. Established in 1987 & committed to dev...

About the Company : Shreenath Chemicals stands as a prominent producer of bulk drugs, drug intermediates, and fine chemicals. Operating from two advanced manufacturing facilities located in M.I.D.C. T...

About the Company : As an internationally renowned outsourcing partner, we offer products and tailor-made service packages that are seamlessly embedded in the value chain of our customers. Our pharmac...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
38
PharmaCompass offers a list of Choline Salicylate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Choline Salicylate manufacturer or Choline Salicylate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Choline Salicylate manufacturer or Choline Salicylate supplier.
PharmaCompass also assists you with knowing the Choline Salicylate API Price utilized in the formulation of products. Choline Salicylate API Price is not always fixed or binding as the Choline Salicylate Price is obtained through a variety of data sources. The Choline Salicylate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cholini salicylas manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cholini salicylas, including repackagers and relabelers. The FDA regulates Cholini salicylas manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cholini salicylas API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cholini salicylas manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cholini salicylas supplier is an individual or a company that provides Cholini salicylas active pharmaceutical ingredient (API) or Cholini salicylas finished formulations upon request. The Cholini salicylas suppliers may include Cholini salicylas API manufacturers, exporters, distributors and traders.
click here to find a list of Cholini salicylas suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cholini salicylas DMF (Drug Master File) is a document detailing the whole manufacturing process of Cholini salicylas active pharmaceutical ingredient (API) in detail. Different forms of Cholini salicylas DMFs exist exist since differing nations have different regulations, such as Cholini salicylas USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cholini salicylas DMF submitted to regulatory agencies in the US is known as a USDMF. Cholini salicylas USDMF includes data on Cholini salicylas's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cholini salicylas USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cholini salicylas suppliers with USDMF on PharmaCompass.
A Cholini salicylas written confirmation (Cholini salicylas WC) is an official document issued by a regulatory agency to a Cholini salicylas manufacturer, verifying that the manufacturing facility of a Cholini salicylas active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Cholini salicylas APIs or Cholini salicylas finished pharmaceutical products to another nation, regulatory agencies frequently require a Cholini salicylas WC (written confirmation) as part of the regulatory process.
click here to find a list of Cholini salicylas suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cholini salicylas as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cholini salicylas API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cholini salicylas as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cholini salicylas and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cholini salicylas NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cholini salicylas suppliers with NDC on PharmaCompass.
Cholini salicylas Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cholini salicylas GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cholini salicylas GMP manufacturer or Cholini salicylas GMP API supplier for your needs.
A Cholini salicylas CoA (Certificate of Analysis) is a formal document that attests to Cholini salicylas's compliance with Cholini salicylas specifications and serves as a tool for batch-level quality control.
Cholini salicylas CoA mostly includes findings from lab analyses of a specific batch. For each Cholini salicylas CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cholini salicylas may be tested according to a variety of international standards, such as European Pharmacopoeia (Cholini salicylas EP), Cholini salicylas JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cholini salicylas USP).
