
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
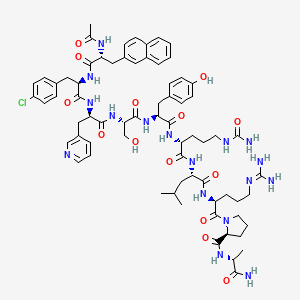

1. Cetrorelix Acetate
2. Cetrorelix Pamoate
3. Cetrotide
4. Lhrh, N-ac-1-nal(2)-2-phe(pcl)-3-pal(3)-6-cit-10-ala-
5. Lhrh, N-acetyl-1-(3-(2-naphthyl)alanyl)-2-(4-chlorophenylalanyl)-3-(3-(3-pyridyl)alanyl)-6-citrulline-10-alanine-
6. N-acetyl-1-(3-(2-naphthyl)alanine)-2-(4-chlorophenylalanine)-3-(3-(3-pyridyl)alanine)-6-citrulline-10-alanine-lhrh
7. Sb 75
8. Sb-75
1. 120287-85-6
2. Cetrotide
3. Cetrorelixum
4. Sb 75
5. Cetrorelixum [inn-latin]
6. Cetrorelix (inn)
7. Cetrorelix [inn]
8. Sb-75
9. Oon1hfz4ba
10. Cetrotide (tn)
11. Chebi:59224
12. 130143-01-0
13. D-alaninamide,n-acetyl-3-(2-naphthalenyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridinyl)-d-alanyl-l-seryl-l-tyrosyl-n5-(aminocarbonyl)-d-ornithyl-l-leucyl-l-arginyl-l-prolyl-
14. Ac-(d-ala[3-(2-naphthyl)])-[d-phe(4-cl)]-(d-ala[3-(3-pyridyl)])-ser-tyr-(d-cit)-leu-arg-pro-d-ala-oh
15. N-acetyl-3-(2-naphthyl)-d-alanyl-p-chloro-d-phenylalanyl-3-(3-pyridyl)-d-alanyl-l-seryl-l-tyrosyl-n(sup 5)-carbamoyl-d-ornithyl-l-leucyl-l-arginyl-l-prolyl-d-alaninamide
16. Sb-075
17. Cetrorelix [inn:ban]
18. Unii-oon1hfz4ba
19. Hsdb 7696
20. Hs-2008
21. Cetrorelix [mi]
22. Cetrorelix [hsdb]
23. Cetrorelix [vandf]
24. Cetrorelix [who-dd]
25. Schembl61331
26. Cetrorelix [ema Epar]
27. Gtpl1190
28. Cetrorelix [orange Book]
29. Chembl1200490
30. Dtxsid7040996
31. Schembl19712202
32. Hy-p0009
33. Vea28785
34. Bdbm50369965
35. Ac-d-nal(2)-d-phe(pcl)-d-pal(3)-ser-tyr-d-cit-leu-arg-pro-d-ala-nh2
36. Akos015994648
37. Db00050
38. Ncgc00481544-01
39. Ncgc00485296-01
40. Ac-28734
41. N-acetyl-3-(2-naphthyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridyl)-d-alanyl-l-seryl-l-tyrosyl-n5-carbomoyl-d-ornithyl-l-leucyl-l-prolyl-d-alaninamide
42. N-acetyl-3-(naphthalen-2-yl)-d-alanyl-4-chloro-d-phenylalanyl-3-(pyridin-3-yl)-d-alanyl-l-seryl-l-tyrosyl-n(5)-carbamoyl-d-ornithyl-l-leucyl-l-arginyl-l-prolyl-d-alaninamide
43. Cs-0012400
44. D07665
45. 287c856
46. Q5065704
47. Y-100040
48. N-acetyl-3-(2-naphthalenyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridinyl)-d-alanyl-l-seryl-l-tyrosyl-n5-(aminocarbonyl)-d-ornithyl-l-leucyl-l-arginyl-l-prolyl-d-alaninamide
| Molecular Weight | 1431.0 g/mol |
|---|---|
| Molecular Formula | C70H92ClN17O14 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 38 |
| Exact Mass | 1429.6698184 g/mol |
| Monoisotopic Mass | 1429.6698184 g/mol |
| Topological Polar Surface Area | 498 Ų |
| Heavy Atom Count | 102 |
| Formal Charge | 0 |
| Complexity | 2840 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cetrotide |
| PubMed Health | Cetrorelix (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Cetrorelix |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.25mg base/ml |
| Market Status | Prescription |
| Company | Emd Serono |
| 2 of 2 | |
|---|---|
| Drug Name | Cetrotide |
| PubMed Health | Cetrorelix (Injection) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Cetrorelix |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 0.25mg base/ml |
| Market Status | Prescription |
| Company | Emd Serono |
Cetrorelix is indicated for the inhibition of premature luteinizing hormone (LH) surges in women undergoing controlled ovarian stimulation.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 353
/EXPL THER/ This randomized, placebo-controlled, single-blind, experimental study was performed on 45 Wistar adult female rats ... . After the peritoneal implantation of endometrial tissue, rats were randomized to three equal intervention groups: (i) control group, (ii) leuprolide group, and (iii) cetrorelix group. Six weeks later, following implant volume measurements (volume-1) by performing a second laparotomy, saline (0.1 mL/rat) was administered subcutaneously to the control group once a week, leuprolide (0.075 mg/kg) subcutaneously to the leuprolide group twice at 4-week intervals and cetrorelix (0.001 mg/rat/day) subcutaneously to the cetrorelix group for 8 weeks. At the end of the treatment, by performing a third laparotomy, implant volumes were remeasured (volume-2) and implants were totally excised for histopathological examination. The volume-1 and volume-2 values within the groups, and stromal and glandular tissue scores between the groups were compared. In both the leuprolide group and the cetrorelix group, volume-2 as compared to volume-1 had significantly reduced (P < 0.01, P < 0.01 respectively), while there was no significant volume change in the control group (P > 0.05). In this group, when compared with the control group, glandular and stromal tissues had significantly lessened (P < 0.01, P < 0.01 respectively). Leuprolide and cetrorelix were found to have similar efficacy in the regression of both the size and the histological structure of experimental endometriotic implants.
PMID:19012701 Altintas D et al; J Obstet Gynaecol Res 34 (6): 1014-9 (2008)
Cetrorelix should be prescribed by health care providers who are experienced in fertility treatment. Before starting treatment with cetrorelix acetate, pregnancy must be excluded.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 354
Elevations in liver function test results including ALT (SGPT), AST (SGOT), gamma-glutamyltransferase (GGT, gamma-glutamyl transpeptidase, GGTP), and alkaline phosphatase up to 3 times the upper limit of normal were reported in 1-2% of patients receiving cetrorelix during controlled ovarian stimulation.
Caution is advised in patients with hypersensitivity to GnRH. Carefully monitor these patients after the first injection. A severe anaphylactic reaction associated with cough, rash, and hypotension was observed in 1 patient after 7 months of treatment with 10 mg/day cetrorelix in a study for an indication unrelated to infertility.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 353
Local site reactions (e.g. redness, erythema, bruising, itching, swelling and pruritus) were reported. Usually, they were of a transient nature, mild intensity and short duration.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
For more Drug Warnings (Complete) data for CETRORELIX (8 total), please visit the HSDB record page.
For the inhibition of premature LH surges in women undergoing controlled ovarian stimulation
FDA Label
Prevention of premature ovulation in patients undergoing a controlled ovarian stimulation, followed by oocyte-pick-up and assisted-reproductive techniques.
In clinical trials, Cetrotide was used with human menopausal gonadotropin (HMG), however, limited experience with recombinant follicule-stimulating hormone (FSH) suggested similar efficacy.
Cetrorelix is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels. Cetrorelix competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner.
Fertility Agents, Female
Compounds which increase the capacity to conceive in females. (See all compounds classified as Fertility Agents, Female.)
Hormone Antagonists
Chemical substances which inhibit the function of the endocrine glands, the biosynthesis of their secreted hormones, or the action of hormones upon their specific sites. (See all compounds classified as Hormone Antagonists.)
H01CC02
H01CC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
H - Systemic hormonal preparations, excl. sex hormones and insulins
H01 - Pituitary and hypothalamic hormones and analogues
H01C - Hypothalamic hormones
H01CC - Anti-gonadotropin-releasing hormones
H01CC02 - Cetrorelix
Absorption
Rapidly absorbed following subcutaneous injection. The mean absolute bioavailability following subcutaneous administration to healthy female subjects is 85%.
Route of Elimination
Following subcutaneous administration of 10 mg cetrorelix to males and females, only unchanged cetrorelix was detected in urine.
Volume of Distribution
1.16 L/kg
Clearance
1.28 ml/minkg [adult healthy female with 3 mg single SC administration]
Following subcutaneous administration of 10 mg cetrorelix to males and females, only unchanged cetrorelix was detected in urine. In 24 hours, cetrorelix and small amounts of the (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples. 2-4% of the dose was eliminated in the urine as unchanged cetrorelix, while 5-10% was eliminated as cetrorelix and the four metabolites in bile. Therefore, only 7-14% of the total dose was recovered as unchanged cetrorelix and metabolites in urine and bile up to 24 hours. The remaining portion of the dose may not have been recovered since bile and urine were not collected for a longer period of time.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
The volume of distribution of Cetrotide following a single intravenous dose of 3 mg is about 1 L/kg. In vitro protein binding to human plasma is 86%. Cetrotide concentrations in follicular fluid and plasma were similar on the day of oocyte pick-up in patients undergoing controlled ovarian stimulation. Following subcutaneous administration of Cetrotide 0.25 mg and 3 mg, plasma concentrations of cetrorelix were below or in the range of the lower limit of quantitation on the day of oocyte pick-up and embryo transfer.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
Cetrotide is rapidly absorbed following subcutaneous injection, maximal plasma concentrations being achieved approximately one to two hours after administration. The mean absolute bioavailability of Cetrotide following subcutaneous administration to healthy female subjects is 85%.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
Pharmacokinetic studies were performed predominantly in rats and dogs. Absorption from the sc injection site was rapid and complete and there were no differences in absorption with regard to sex or species. A linear relationship between dose and plasma AUC was evident. Distribution of cetrorelix was rapid. Main target organs were the kidney, liver, small intestine and organs containing the luteinising hormone releasing hormone (LHRH) receptor (pituitary gland, ovaries). Plasma protein binding amounted to 86%. Elimination from most tissues was rapid and occurred predominantly within 48 hr. ... Cetrorelix crosses the placenta only to a low extent. The distribution of cetrorelix or its metabolites into milk was not investigated. Cetrorelix is excreted unchanged into urine and after metabolism by peptidases into bile. ... Studies in healthy volunteers indicate that cetrorelix is excreted in a similar manner in humans, rats and dogs.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
Following subcutaneous injection, the absolute bioavailability of cetrorelix was approximately 85% in both males and females. The apparent volume of distribution was 1.16 + or - 0.29 L/kg in females and 1.02 + or - 0.33 L/kg in males. The terminal half-life was about 10 hours after iv and 30 hours after subcutaneous injection with a trend towards lower values in female. Protein binding in human plasma was around 85%. Linear pharmacokinetics were observed following both single (0.25, 0.5 and 1.00 mg) and multiple dose administration (0.25 to 1.00 mg). The pharmacokinetics were linear up to a 3 mg dose.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
In in vitro studies, cetrorelix was stable against phase I- and phase II-metabolism. Cetrorelix was transformed by peptidases, and the (1-4) peptide was the predominant metabolite.
The main metabolite of cetrorelix in the rat bile was identified as being the heptapeptide (1-7). The metabolite was pharmacologically inactive in rats, in terms of testosterone suppression.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
After subcutaneous administration of 10 mg Cetrotide to females and males, Cetrotide and small amounts of (1-9), (1-7), (1-6), and (1-4) peptides were found in bile samples over 24 hours. In in vitro studies, Cetrotide was stable against phase I- and phase II-metabolism. Cetrotide was transformed by peptidases, and the (1-4) peptide was the predominant metabolite.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
~62.8 hours
In humans, the terminal half-life values after iv and sc administration were 8-9 hr and 24-40 hr, respectively.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
The terminal half-lives in rats after iv and sc administration were 1-2 hr and 7-14 hr, respectively ... .
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
Elimination half life: Single 3 mg dose: 62.8 hr (38.2-108 hr); Single 0.25 mg dose: 5.0 hr (2.4-48.8 hr); 0.25 mg daily for 14 days: dose: 20.6 hr (4.1-179.3 hr) /From table/
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
Half-lives of greater than or equal to 100 hr were observed mainly in organs of elimination (liver, kidney), spleen and in the organs containing LHRH binding sites.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
Cetrorelix binds to the gonadotropin releasing hormone receptor and acts as a potent inhibitor of gonadotropin secretion. It competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner.
Cetrotide competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner. The onset of LH suppression is approximately one hour with the 3 mg dose and two hours with the 0.25 mg dose. This suppression is maintained by continuous treatment and there is a more pronounced effect on LH than on FSH. An initial release of endogenous gonadotropins has not been detected with Cetrotide, which is consistent with an antagonist effect.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels.
US Natl Inst Health; DailyMed. Current Medication Information for Cetrotide (Cetrorelix acetate) (April 2008). Available from, as of February 9, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7634
The mechanisms through which luteinizing hormone (LH)-releasing hormone (LHRH) antagonists suppress pituitary gonadotroph functions and LHRH-receptor (LHRH-R) expression are incompletely understood. Consequently, we investigated the direct effect of LHRH antagonist cetrorelix in vitro on the expression of the pituitary LHRH-R gene and its ability to counteract the exogenous LHRH and the agonist triptorelin in the regulation of this gene. We also compared the effects of chronic administration of cetrorelix and triptorelin on the LHRH-R mRNA level and gonadotropin secretion in ovariectomized (OVX) and normal female rats. The exposure of pituitary cells in vitro to 3-min pulses of 1 nM LHRH or 0.1 nM triptorelin for 5 hr increased the LHRH-R mRNA level by 77-88%. Continuous perfusion of the cells with 50 nM cetrorelix did not cause any significant changes, but prevented the stimulatory effect of LHRH pulses on the receptor mRNA expression. In OVX rats, 10 days after administration of a depot formulation of cetrorelix, releasing 100 microg of peptide daily, the elevated LHRH-R mRNA level was decreased by 73%, whereas daily injection of 100 microg of triptorelin caused a 41% suppression. In normal female rats, cetrorelix treatment suppressed the LHRH-R mRNA level by 33%, but triptorelin increased it by 150%. The highly elevated serum LH levels in OVX rats and the normal LH concentration of cycling rats were rapidly and completely suppressed by cetrorelix. Triptorelin decreased the serum LH in OVX rats to the precastration level, but had no effect on basal LH in normal rats. Our results confirm that LHRH antagonists, such as cetrorelix, inhibit the gene expression of pituitary LHRH-R indirectly, by counteracting the stimulatory effect of LHRH. A rapid suppression of serum LH by LHRH antagonists would be advantageous in the treatment of sex hormone-dependent tumors and other conditions.
PMID:11593037 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC59791 Kovacs M, Schally AV; Proc Natl Acad Sci U S A 98 (21): 12197-202 (2001)
Cetrorelix binds with high affinity to luteinising hormone releasing hormone (LHRH) receptors, and labelled LHRH was shown to be displaced from the two classes of LHRH receptors by the compound. In vitro, cetrorelix dose-dependently inhibited the LHRH-mediated LH release in perfused rat pituitary cell systems. Further, in a recently established in vitro assay, cetrorelix proved to be a high affinity antagonist at the LHRH-receptor and dose-dependently blocked the signal transduction induced by LHRH.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Cetrotide, Scientific Discussion (2003). Available from, as of February 15, 2009: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Cetrotide/297998en6.pdf
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, has established itself as a reliable partner in the development and manufacturing of niche and complex pharmaceutical products. With 4 USFDA and EU-appro...
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
About the Company : Established in 1982, ChemWerth is a US-headquartered full-service generic active pharmaceutical ingredient (API) development and supply company. ChemWerth offers cGMP-quality APIs ...
About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
About the Company : Omgene Life Sciences Private Limited is an R&D-driven biopharmaceutical company specializing in biopharmaceuticals, peptides, semi-synthetic, and synthetic actives. As a vertically...
About the Company : ALP Pharm began to manufacture some APIs in the partner's facilities in 2007. Some of our APIs have been approved by EDQM and US FDA. Our professional regulatory compliance experts...

About the Company : Eagle Chemical was founded in the year 1978 by Gautam Shah a man with an enterprising zeal & a vision to make a mark in the nascent pharmaceutical raw material manufacturing sector...

About the Company : Hybio is a hi-tech enterprise specializing in the R&D and manufacturing of diverse peptide products. Founded in 1998, after decades of effort, we have grown to be one of the larges...

About the Company : Sinopep leads the industry in therapeutic peptides, supplying top-demand APIs like Albuvirtide, Lanreotide, Semaglutide, and more. Renowned for high-quality peptide APIs and effici...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
60
PharmaCompass offers a list of Cetrorelix Acetate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cetrorelix Acetate manufacturer or Cetrorelix Acetate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cetrorelix Acetate manufacturer or Cetrorelix Acetate supplier.
PharmaCompass also assists you with knowing the Cetrorelix Acetate API Price utilized in the formulation of products. Cetrorelix Acetate API Price is not always fixed or binding as the Cetrorelix Acetate Price is obtained through a variety of data sources. The Cetrorelix Acetate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CETRORELIX (2) manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CETRORELIX (2), including repackagers and relabelers. The FDA regulates CETRORELIX (2) manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CETRORELIX (2) API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CETRORELIX (2) manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CETRORELIX (2) supplier is an individual or a company that provides CETRORELIX (2) active pharmaceutical ingredient (API) or CETRORELIX (2) finished formulations upon request. The CETRORELIX (2) suppliers may include CETRORELIX (2) API manufacturers, exporters, distributors and traders.
click here to find a list of CETRORELIX (2) suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CETRORELIX (2) DMF (Drug Master File) is a document detailing the whole manufacturing process of CETRORELIX (2) active pharmaceutical ingredient (API) in detail. Different forms of CETRORELIX (2) DMFs exist exist since differing nations have different regulations, such as CETRORELIX (2) USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CETRORELIX (2) DMF submitted to regulatory agencies in the US is known as a USDMF. CETRORELIX (2) USDMF includes data on CETRORELIX (2)'s chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CETRORELIX (2) USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CETRORELIX (2) suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The CETRORELIX (2) Drug Master File in Japan (CETRORELIX (2) JDMF) empowers CETRORELIX (2) API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the CETRORELIX (2) JDMF during the approval evaluation for pharmaceutical products. At the time of CETRORELIX (2) JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of CETRORELIX (2) suppliers with JDMF on PharmaCompass.
A CETRORELIX (2) written confirmation (CETRORELIX (2) WC) is an official document issued by a regulatory agency to a CETRORELIX (2) manufacturer, verifying that the manufacturing facility of a CETRORELIX (2) active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting CETRORELIX (2) APIs or CETRORELIX (2) finished pharmaceutical products to another nation, regulatory agencies frequently require a CETRORELIX (2) WC (written confirmation) as part of the regulatory process.
click here to find a list of CETRORELIX (2) suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing CETRORELIX (2) as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for CETRORELIX (2) API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture CETRORELIX (2) as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain CETRORELIX (2) and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a CETRORELIX (2) NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of CETRORELIX (2) suppliers with NDC on PharmaCompass.
CETRORELIX (2) Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CETRORELIX (2) GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CETRORELIX (2) GMP manufacturer or CETRORELIX (2) GMP API supplier for your needs.
A CETRORELIX (2) CoA (Certificate of Analysis) is a formal document that attests to CETRORELIX (2)'s compliance with CETRORELIX (2) specifications and serves as a tool for batch-level quality control.
CETRORELIX (2) CoA mostly includes findings from lab analyses of a specific batch. For each CETRORELIX (2) CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CETRORELIX (2) may be tested according to a variety of international standards, such as European Pharmacopoeia (CETRORELIX (2) EP), CETRORELIX (2) JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CETRORELIX (2) USP).
