
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers
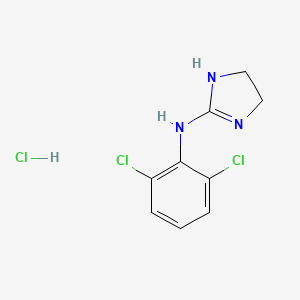

1. Catapres
2. Catapresan
3. Catapressan
4. Chlophazolin
5. Clofelin
6. Clofenil
7. Clonidine
8. Clonidine Dihydrochloride
9. Clonidine Monohydrobromide
10. Clonidine Monohydrochloride
11. Clopheline
12. Dihydrochloride, Clonidine
13. Dixarit
14. Gemiton
15. Hemiton
16. Hydrochloride, Clonidine
17. Isoglaucon
18. Klofelin
19. Klofenil
20. M 5041t
21. M-5041t
22. M5041t
23. Monohydrobromide, Clonidine
24. Monohydrochloride, Clonidine
25. St 155
26. St-155
27. St155
1. 4205-91-8
2. Clonidine Hcl
3. 2-(2,6-dichloroanilino)-2-imidazoline Hydrochloride
4. Kapvay
5. N-(2,6-dichlorophenyl)-4,5-dihydro-1h-imidazol-2-amine Hydrochloride
6. St-155
7. Catapresan
8. Jenloga
9. Clonidine (hydrochloride)
10. Nsc-756699
11. 2-(2,6-dichlorophenylamino)-2-imidazoline Hydrochloride
12. W76i6xxf06
13. Dichloranilino Imidazolin
14. 2-((2,6-dichlorophenyl)imino)imidazolidine Monohydrochloride
15. Benzenamine, 2,6-dichloro-n-2-imidazolidinylidene-, Monohydrochloride
16. Chlophazolin
17. Dispaclonidin
18. Normopresan
19. Atensina
20. Barclyd
21. Capresin
22. Caprysin
23. Catanidin
24. Clofelin
25. Clonilou
26. Clonisin
27. Clonistada
28. Dixarit
29. Edolglau
30. Glausine
31. Haemiton
32. Ipotensium
33. Isoglaucon
34. Katapresan
35. Klophelin
36. Iporel
37. 1h-imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro- [cas]
38. Apo-clonidine
39. Clonidil-riker
40. Novo-clonidine
41. Clonid-ophal
42. Nu-clonidine
43. N-(2,6-dichlorophenyl)-4,5-dihydro-1h-imidazol-2-amine Hcl
44. 1h-imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-, Monohydrochloride (9ci)
45. 2-[(2,6-dichlorophenyl)amino-2-imidazoline Hydrochloride
46. Bapresan
47. Clonicel
48. Trialox
49. Clonidine Monohydrochloride
50. Smr000466276
51. 205c918
52. Sr-01000075246
53. Einecs 224-121-5
54. Cas-4205-91-8
55. Mfcd00036705
56. N-(2,6-dichlorophenyl)imidazolidin-2-imine;hydrochloride
57. Unii-w76i6xxf06
58. Clonidine-hcl
59. Sr-01000759380
60. Prestwick_73
61. Catapres (tn)
62. 4,5-dihydro-
63. 2-((2,6-dichlorophenyl)amino)-2-imidazoline Hydrochloride
64. 2-imidazoline, 2-(2,6-dichloroanilino)-, Monohydrochloride
65. 2-(2,6-dichlorophenylamino)-2-imidazolin Hydrochlorid [german]
66. Benzenamine, 2,6-dichloro-n-2-imidazolidinylidene-, Hydrochloride
67. Clonidine Hydrochloride [usan:usp:ban:jan]
68. 1h-imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-, Monohydrochloride
69. Clonidinehydrochloride
70. N-(2,6-dichlorophenyl)-
71. Chembl1705
72. Clonidine Hydrochloride,(s)
73. Dsstox_cid_24670
74. Dsstox_rid_80388
75. Dsstox_gsid_44670
76. Schembl40751
77. Mls000758256
78. Mls001424222
79. Clonidine Hydrochloride, Solid
80. 2-(2,6-dichlorophenylamino)-2-imidazolin Hydrochlorid
81. Chebi:3758
82. Dtxsid8044670
83. Hy-b0409a
84. Clonidine Hydrochloride (catapres)
85. Hms1568b18
86. Hms3414h03
87. Hms3678h03
88. Pharmakon1600-01500198
89. Bcp14375
90. Clonidine Hydrochloride [mi]
91. Tox21_301552
92. Tox21_500268
93. Bdbm50020341
94. Ccg-40087
95. Clonidine Hydrochloride (jp17/usp)
96. Clonidine Hydrochloride [jan]
97. N-(2,6-dichlorophenyl)-4,5-dihydro-1h-imidazol-2-amine;hydrochloride
98. Nsc756699
99. S2458
100. Clonidine Hydrochloride [usan]
101. Akos005267207
102. Akos024458609
103. Akos028114993
104. Ccg-101051
105. Ccg-212540
106. Ccg-229466
107. Clonidine Hydrochloride [mart.]
108. Clonidine Hydrochloride [vandf]
109. Ks-1253
110. Lp00268
111. Nc00301
112. Nsc 756699
113. Clonidine Hydrochloride [usp-rs]
114. Clonidine Hydrochloride [who-dd]
115. Ncgc00093726-01
116. Ncgc00093726-02
117. Ncgc00180902-01
118. Ncgc00256172-01
119. Ncgc00260953-01
120. Ac-10306
121. Ac-29742
122. Clonidine Hydrochloride, >=98.0% (tlc)
123. Clonidine Hydrochloride [ep Impurity]
124. Clonidine Hydrochloride [orange Book]
125. D1353
126. Eu-0100268
127. Ft-0601096
128. Ft-0665123
129. Sw197681-3
130. Clonidine Hydrochloride [ep Monograph]
131. C 7897
132. Clonidine Hydrochloride [usp Monograph]
133. D00604
134. D81843
135. Clorpres Component Clonidine Hydrochloride
136. A825736
137. Clonidine Hydrochloride 100 Microg/ml In Methanol
138. Combipres Component Clonidine Hydrochloride
139. Clonidine Hydrochloride Component Of Clorpres
140. Q-200880
141. Sr-01000075246-1
142. Sr-01000075246-3
143. Sr-01000759380-5
144. Clonidine Hydrochloride (catapres) [largely Precursor]
145. Clonidine Hydrochloride Component Of Combipres
146. Q27292429
147. 2-[(2,6-dichlorophenyl)imino]imidazolidine Hydrochloride
148. Clonidine Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
149. Clonidine Hydrochloride, British Pharmacopoeia (bp) Reference Standard
150. Clonidine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
151. Clonidine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
152. 1h-imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-, Hydrochloride (1:1)
153. N-[2,6-bis(chloranyl)phenyl]-4,5-dihydro-1h-imidazol-2-amine Hydrochloride
| Molecular Weight | 266.6 g/mol |
|---|---|
| Molecular Formula | C9H10Cl3N3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 264.994030 g/mol |
| Monoisotopic Mass | 264.994030 g/mol |
| Topological Polar Surface Area | 36.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 222 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Clonidine hydrochloride |
| PubMed Health | Clonidine |
| Drug Classes | Analgesic, Anesthetic Adjunct, Antihypertensive, Antimigraine, Cardiovascular Agent, Central Nervous System Agent, Diagnostic Agent, Pheochromocytoma |
| Drug Label | Clonidine hydrochloride, USP is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg, and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base.The fo... |
| Active Ingredient | Clonidine hydrochloride |
| Dosage Form | Tablet, extended release; Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 0.2mg; 1mg/10ml (0.1mg/ml); 0.3mg; 5mg/10ml (0.5mg/ml); 0.1mg |
| Market Status | Prescription |
| Company | Exela Pharma Scs; Anchen Pharms; Actavis Elizabeth; Fresenius Kabi Usa; Unichem; Mutual Pharm; Vintage; Alembic Pharms; Hikma Farmaceutica; Luitpold; Sun Pharm Inds; Zydus Pharms Usa; Carlsbad; Mylan; Impax Labs; Dava Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Kapvay |
| Drug Label | KAPVAY (clonidine hydrochloride) extended-release is a centrally acting alpha2-adrenergic agonist available as 0.1 mg or 0.2 mg extended-release tablets for oral administration. Each 0.1 mg and 0.2 mg tablet is equivalent to 0.087 mg and 0.174 mg, re... |
| Active Ingredient | Clonidine hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 0.1mg; 0.2mg |
| Market Status | Prescription |
| Company | Concordia Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Clonidine hydrochloride |
| PubMed Health | Clonidine |
| Drug Classes | Analgesic, Anesthetic Adjunct, Antihypertensive, Antimigraine, Cardiovascular Agent, Central Nervous System Agent, Diagnostic Agent, Pheochromocytoma |
| Drug Label | Clonidine hydrochloride, USP is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg, and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base.The fo... |
| Active Ingredient | Clonidine hydrochloride |
| Dosage Form | Tablet, extended release; Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 0.2mg; 1mg/10ml (0.1mg/ml); 0.3mg; 5mg/10ml (0.5mg/ml); 0.1mg |
| Market Status | Prescription |
| Company | Exela Pharma Scs; Anchen Pharms; Actavis Elizabeth; Fresenius Kabi Usa; Unichem; Mutual Pharm; Vintage; Alembic Pharms; Hikma Farmaceutica; Luitpold; Sun Pharm Inds; Zydus Pharms Usa; Carlsbad; Mylan; Impax Labs; Dava Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Kapvay |
| Drug Label | KAPVAY (clonidine hydrochloride) extended-release is a centrally acting alpha2-adrenergic agonist available as 0.1 mg or 0.2 mg extended-release tablets for oral administration. Each 0.1 mg and 0.2 mg tablet is equivalent to 0.087 mg and 0.174 mg, re... |
| Active Ingredient | Clonidine hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 0.1mg; 0.2mg |
| Market Status | Prescription |
| Company | Concordia Pharms |
Sedation
Adrenergic alpha-2 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Agonists.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Sympatholytics
Drugs that inhibit the actions of the sympathetic nervous system by any mechanism. The most common of these are the ADRENERGIC ANTAGONISTS and drugs that deplete norepinephrine or reduce the release of transmitters from adrenergic postganglionic terminals (see ADRENERGIC AGENTS). Drugs that act in the central nervous system to reduce sympathetic activity (e.g., centrally acting alpha-2 adrenergic agonists, see ADRENERGIC ALPHA-AGONISTS) are included here. (See all compounds classified as Sympatholytics.)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Catapresan
Dosage Form : SOLUTION FOR INJECTION
Dosage Strength : 150 MICROGRAM / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Catapresan
Dosage Form : Clonidine 300Mcg 30 Joined' Oral Use
Dosage Strength : 30 CPR 300 mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Catapresan Tts
Dosage Form : Clonidine 5Mg 2 Plasters Transdermal Use
Dosage Strength : 2 plasters transd 5 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Catapresan
Dosage Form :
Dosage Strength : 30 Cpr 150 Mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Catapresan
Dosage Form : Clonidine 0.15Mg 1Ml 5 Units Parenteral Use
Dosage Strength : 5 VIALS SC IM EV 0.15 mg 1 ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Catapresan
Dosage Form : Antic-calc Tablet, drasjert
Dosage Strength : 25 mcg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Catapres
Dosage Form : SOLUTION FOR INJECTION
Dosage Strength : 150 MICROGRAM / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Regulatory Info :
Registration Country : Sweden
Brand Name : Catapres
Dosage Form : SOLUTION FOR INJECTION
Dosage Strength : 150 MICROGRAM / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Sweden
Brand Name : Catapres
Dosage Form : SOLUTION FOR INJECTION
Dosage Strength : 150 MICROGRAM / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Sweden
Brand Name : Catapres
Dosage Form : SOLUTION FOR INJECTION
Dosage Strength : 150 MICROGRAM / ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : Espheres EM can be used as an inert base for modified release formulations promoting uniformity of release profile.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Instacoat CFC is an HPMC & xylitol-based high-speed optimized chewable and crunchy film coating material.
Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : Instamodel Blend is used to provide extended release from oral dosage forms.
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175 µm, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Application : Fillers, Diluents & Binders
Excipient Details : KoVidone® K25 is used as a low viscosity wet binder in solid dosage forms such as capsules and tablets.
Pharmacopoeia Ref : USP/NF, EP, JP, KP, IP, BP
Technical Specs : NA
Ingredient(s) : Polyvinylpyrrolidone
Brand Name : Lactose Monohydrate
Application : Parenteral
Excipient Details : Lactose monohydrate is used as a diluent in inhalation and lyophilized preparations.
Application : Fillers, Diluents & Binders
Excipient Details : KoVidone® K30 is used as a binder for tablets and capsules.
Pharmacopoeia Ref : USP/NF, EP, JP, KP, IP, BP
Technical Specs : NA
Ingredient(s) : Polyvinylpyrrolidone K30
Grade : Oral
Category : Co-Processed Excipients, Fillers, Diluents & Binders
Application : Co-Processed Excipients, Fillers, Diluents & Binders
Excipient Details : ProBlend (SMCC) is a co-processed excipient consists of microcrystalline cellulose & colloidal silicon dioxide, used as a diluent & binder in OSDs.
Pharmacopoeia Ref : USP-NF, DMF, EXCiPAT, KOSHER, ...
Technical Specs : NA
Ingredient(s) : Silicified Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : Titanium dioxide PRETIOX AV01FG
Application : Coating Systems & Additives, Coloring Agents
Excipient Details : Titanium dioxide Pretiox AV01FG is used as a coloring and coating agent in oral solid dosage forms such as capsules, tablets, granules, and pellets.
Pharmacopoeia Ref : Fami-QS, Kosher, Halal, OHSAS ...
Technical Specs : Ti 59.95% and O 40.05%
Ingredient(s) : Titanium Dioxide
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Coating Systems & Additives
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Emulsion, Tablet
Grade : Oral, Ophthalmic
Category : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Excipient Details : Hydroxypropyl Methyl Cellulose is used as a film-former, disintegrant, thickener, and emulsifier in tablets, emulsions, and ophthalmic formulations.
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Sustained Release Tablet Matrix
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Starch
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Capsule
Grade : Oral and Inhalation
Category : API Stability Enhancers, Vegetarian Capsules
Application : API Stability Enhancers, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : "Water content – less than 9%, can be customized; Size # 00el -...
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Application : Controlled & Modified Release, Vegetarian Capsules
Pharmacopoeia Ref : Complies with relevant Europea...
Technical Specs : Water content – less than 6%; Size #0
Ingredient(s) : HPMC AS
Dosage Form : Capsule
Grade : Oral
Category : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Application : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : Size # 0-1
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
12
PharmaCompass offers a list of Clonidine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Clonidine manufacturer or Clonidine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Clonidine manufacturer or Clonidine supplier.
PharmaCompass also assists you with knowing the Clonidine API Price utilized in the formulation of products. Clonidine API Price is not always fixed or binding as the Clonidine Price is obtained through a variety of data sources. The Clonidine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CATAPRES-TTS-2 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CATAPRES-TTS-2, including repackagers and relabelers. The FDA regulates CATAPRES-TTS-2 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CATAPRES-TTS-2 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CATAPRES-TTS-2 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CATAPRES-TTS-2 supplier is an individual or a company that provides CATAPRES-TTS-2 active pharmaceutical ingredient (API) or CATAPRES-TTS-2 finished formulations upon request. The CATAPRES-TTS-2 suppliers may include CATAPRES-TTS-2 API manufacturers, exporters, distributors and traders.
click here to find a list of CATAPRES-TTS-2 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CATAPRES-TTS-2 DMF (Drug Master File) is a document detailing the whole manufacturing process of CATAPRES-TTS-2 active pharmaceutical ingredient (API) in detail. Different forms of CATAPRES-TTS-2 DMFs exist exist since differing nations have different regulations, such as CATAPRES-TTS-2 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CATAPRES-TTS-2 DMF submitted to regulatory agencies in the US is known as a USDMF. CATAPRES-TTS-2 USDMF includes data on CATAPRES-TTS-2's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CATAPRES-TTS-2 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CATAPRES-TTS-2 suppliers with USDMF on PharmaCompass.
A CATAPRES-TTS-2 CEP of the European Pharmacopoeia monograph is often referred to as a CATAPRES-TTS-2 Certificate of Suitability (COS). The purpose of a CATAPRES-TTS-2 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of CATAPRES-TTS-2 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of CATAPRES-TTS-2 to their clients by showing that a CATAPRES-TTS-2 CEP has been issued for it. The manufacturer submits a CATAPRES-TTS-2 CEP (COS) as part of the market authorization procedure, and it takes on the role of a CATAPRES-TTS-2 CEP holder for the record. Additionally, the data presented in the CATAPRES-TTS-2 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the CATAPRES-TTS-2 DMF.
A CATAPRES-TTS-2 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. CATAPRES-TTS-2 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of CATAPRES-TTS-2 suppliers with CEP (COS) on PharmaCompass.
A CATAPRES-TTS-2 written confirmation (CATAPRES-TTS-2 WC) is an official document issued by a regulatory agency to a CATAPRES-TTS-2 manufacturer, verifying that the manufacturing facility of a CATAPRES-TTS-2 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting CATAPRES-TTS-2 APIs or CATAPRES-TTS-2 finished pharmaceutical products to another nation, regulatory agencies frequently require a CATAPRES-TTS-2 WC (written confirmation) as part of the regulatory process.
click here to find a list of CATAPRES-TTS-2 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing CATAPRES-TTS-2 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for CATAPRES-TTS-2 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture CATAPRES-TTS-2 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain CATAPRES-TTS-2 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a CATAPRES-TTS-2 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of CATAPRES-TTS-2 suppliers with NDC on PharmaCompass.
CATAPRES-TTS-2 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CATAPRES-TTS-2 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CATAPRES-TTS-2 GMP manufacturer or CATAPRES-TTS-2 GMP API supplier for your needs.
A CATAPRES-TTS-2 CoA (Certificate of Analysis) is a formal document that attests to CATAPRES-TTS-2's compliance with CATAPRES-TTS-2 specifications and serves as a tool for batch-level quality control.
CATAPRES-TTS-2 CoA mostly includes findings from lab analyses of a specific batch. For each CATAPRES-TTS-2 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CATAPRES-TTS-2 may be tested according to a variety of international standards, such as European Pharmacopoeia (CATAPRES-TTS-2 EP), CATAPRES-TTS-2 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CATAPRES-TTS-2 USP).
