

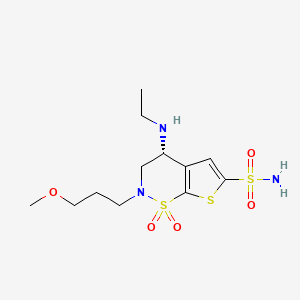

1. (r)-4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2h-thieno(3,2-e)-1,2-thiazine-6-sulfonamide 1,1-dioxide
2. Azopt
1. 138890-62-7
2. Azopt
3. Al-4862
4. Al 4862
5. (4r)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-3,4-dihydrothieno[3,2-e]thiazine-6-sulfonamide
6. (4r)-4-(ethylamino)-2-(3-methoxypropyl)-3,4-dihydro-2h-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
7. 138890-50-3
8. Chebi:3176
9. (r)-4-(ethylamino)-2-(3-methoxypropyl)-3,4-dihydro-2h-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
10. Nsc-760050
11. (+)-4-ethylamino-3,4-dihydro-2-(methoxy)propyl-2h-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide
12. (r)-4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2h-thieno(3,2-e)-1,2-thiazine-6-sulfonamide 1,1-dioxide
13. (r)-4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2h-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide
14. 2h-thieno(3,2-e)-1,2-thiazine-6-sulfonamide, 4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-, 1,1-dioxide, (r)-
15. Brinzolamide [usan]
16. Mfcd08067749
17. Dsstox_cid_25531
18. Dsstox_rid_80934
19. Dsstox_gsid_45531
20. 2h-thieno[3,2-e]-1,2-thiazine-6-sulfonamide, 4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-, 1,1-dioxide, (4r)-
21. Bz1
22. 9451z89515
23. (4r)-4-(ethylamino)-3,4-dihydro-2-(3-methoxypropyl)-2h-thieno[3,2-e]-1,2-thiazine-6-sulfonamide 1,1-dioxide
24. Brinzolamide (brz)
25. Azopt (tn)
26. Brinzolamide (jan/usp/inn)
27. 3znc
28. Ncgc00016979-01
29. Brinzolamide [usan:usp:inn:ban]
30. Unii-9451z89515
31. Brinzolamide- Bio-x
32. Cas-138890-62-7
33. Prestwick0_000365
34. Prestwick1_000365
35. Prestwick2_000365
36. Prestwick3_000365
37. Brinzolamide [mi]
38. Brinzolamide [inn]
39. Brinzolamide [jan]
40. Brinzolamide [vandf]
41. Schembl24636
42. Brinzolamide [mart.]
43. Bspbio_000489
44. Mls002153787
45. Bidd:gt0039
46. Brinzolamide [usp-rs]
47. Brinzolamide [who-dd]
48. Spbio_002410
49. Amy372
50. Bpbio1_000539
51. Chembl220491
52. Gtpl6797
53. Brinzolamide [ema Epar]
54. Dtxsid6045531
55. Bdbm10885
56. Brinzolamide, >=98% (hplc)
57. Brinzolamide [orange Book]
58. Hms1569i11
59. Hms2096i11
60. Hms2234k06
61. Hms3713i11
62. Hms3885i11
63. Brinzolamide [usp Impurity]
64. Azarga Component Brinzolamide
65. Bcp22330
66. Hy-b0588
67. Zinc3953037
68. Brinzolamide [usp Monograph]
69. Tox21_110722
70. S3178
71. Akos005145708
72. Simbrinza Component Brinzolamide
73. Tox21_110722_1
74. Ac-5277
75. Brinzolamide Component Of Azarga
76. Ccg-220365
77. Ccg-222516
78. Db01194
79. Nsc 760050
80. Ncgc00179542-03
81. Ncgc00179542-09
82. Ncgc00179542-10
83. As-35084
84. Bb164262
85. Brinzolamide Component Of Simbrinza
86. Smr001233169
87. Ab00513824
88. B4258
89. Sw197152-3
90. Al4862;al 4862;al-4862
91. C07760
92. D00652
93. Ab00513824_06
94. Q411517
95. Sr-01000838832
96. Q-200751
97. Sr-01000838832-2
98. Brd-k74913225-001-03-3
99. Brinzolamide, United States Pharmacopeia (usp) Reference Standard
100. (4r)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-2h,3h,4h-1$l^{6},7,2-thieno[3,2-e][1$l^{6},2]thiazine-6-sulfonamide
101. (4r)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-2h,3h,4h-1$l^{6},7,2-thieno[3,2-e][1,2]thiazine-6-sulfonamide
102. (4r)-4-(ethylamino)-2-(3-methoxypropyl)-3,4-dihydro-2h-thieno[3,2-e][1,2]thiazine-6-sulfonamide-1,1-dioxide
103. (4r)-4-ethylamino-2-(3-methoxypropyl)-1,1-dioxo-3,4-dihydrothieno[4,5-e]thiazine-6-sulfonamide
104. (5r)-5-ethylamino-3-(3-methoxypropyl)-2,2-dioxo-2,9-dithia-3-azabicyclo[4.3.0]nona-1(6)7-diene-8-sulfonamide
105. (r)-3,4-dihydro-4-ethylamino-2-(3-methoxypropyl)-2h-thieno[3,2-e]-1,2-thiazine-6-sulfonamide-1,1-dioxide
| Molecular Weight | 383.5 g/mol |
|---|---|
| Molecular Formula | C12H21N3O5S3 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 383.06433430 g/mol |
| Monoisotopic Mass | 383.06433430 g/mol |
| Topological Polar Surface Area | 164 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 598 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Azopt |
| PubMed Health | Brinzolamide (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | AZOPT (brinzolamide ophthalmic suspension) 1% contains a carbonic anhydrase inhibitor formulated for multidose topical ophthalmic use. Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1... |
| Active Ingredient | Brinzolamide |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Azopt |
| PubMed Health | Brinzolamide (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | AZOPT (brinzolamide ophthalmic suspension) 1% contains a carbonic anhydrase inhibitor formulated for multidose topical ophthalmic use. Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1... |
| Active Ingredient | Brinzolamide |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Alcon Pharms |
For the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
FDA Label
Azopt is indicated to decrease elevated intraocular pressure in:
- ocular hypertension;
- open-angle glaucomaas monotherapy in adult patients unresponsive to beta-blockers or in adult patients in whom beta-blockers are contraindicated, or as adjunctive therapy to beta-blockers or prostaglandin analogues.
Used in the treatment of glaucoma, brinzolamide inhibits aqueous humor formation and reduces elevated intraocular pressure. Elevated intraocular pressure is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss. Brinzolamide can decrease intraocular pressure by approximately 16-19% in patients with elevated intraocular pressure.
Carbonic Anhydrase Inhibitors
A class of compounds that reduces the secretion of H+ ions by the proximal kidney tubule through inhibition of CARBONIC ANHYDRASES. (See all compounds classified as Carbonic Anhydrase Inhibitors.)
S01EC04
S01EC04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EC - Carbonic anhydrase inhibitors
S01EC04 - Brinzolamide
Absorption
Absorbed into systemic circulation following topical ocular application
Ophthalmic
111 days
Brinzolamide is a highly specific inhibitor of CA-II, which is the main CA isoenzyme involved in the secretion of aqueous humor. Inhibition of CA in the ciliary process of the eye slows the formation of bicarbonate, and reduces sodium and fluid transport. This results in a reduction in the rate of aqueous humor secretion and the intraocular pressure. Brinzolamide is absorbed systemically following topical ocular administration. Since it has a high affinity for CA-II, brinzolamide binds extensively to red blood cells, where CA-II is primarily found. As sufficient CA-II activity remains, adverse effects resulting from the systemic inhibition of CA by brinzolamide are not observed. The metabolite N-desethyl brinzolamide is also formed. This metabolite binds to CA and accumulates in red blood cells as well. In the presence of brinzolamide, the metabolite binds mainly to carbonic anhydrase I (CA-I).
