
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. C.i. 45350
2. Colircusi Fluoresceina
3. D And C Yellow No. 7
4. D And C Yellow No. 8
5. Diofluor
6. Dipotassium Salt, Fluorescein
7. Disodium Fluorescein
8. Disodium Salt, Fluorescein
9. Fluor I Strip A.t.
10. Fluor-i-strip A.t.
11. Fluorescine Sodique Faure
12. Fluorescein
13. Fluorescein Dipotassium Salt
14. Fluorescein Disodium Salt
15. Fluorescein Monosodium Salt
16. Fluorescein Sodium, Minims
17. Fluorescein, Disodium
18. Fluorescein, Sodium
19. Fluoresceina, Colircusi
20. Fluoresceine, Minims
21. Fluorescite
22. Fluorets
23. Ful Glo
24. Ful-glo
25. Funduscein
26. Minims Fluorescein Sodium
27. Minims Fluoresceine
28. Minims Stains
29. Monosodium Salt, Fluorescein
30. Optifluor Diba
31. Sodium Fluorescein
32. Sodium, Fluorescein
33. Uranine
1. Fluorescein Disodium Salt
2. Soluble Fluorescein
3. Uranin
4. Sodium Fluorescein
5. Fluorescein, Disodium Salt
6. C.i. Acid Yellow 73
7. Resorcinol Phthalein Sodium
8. Uranine Wss
9. C.i. 45350
10. Fluorescein, Soluble
11. Ki202
12. 93x55pe38x
13. Disodium;2-(3-oxido-6-oxoxanthen-9-yl)benzoate
14. Ki202(1)
15. Ki202(2)
16. Disodium 2-(6-oxido-3-oxo-3h-xanthen-9-yl)benzoate
17. Ci 45350
18. Aizen Uranine
19. Calcocid Uranine B4315
20. Spiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one, 3',6'-dihydroxy-, Sodium Salt (1:2)
21. Spiro(isobenzofuran-1(3h),9'-(9h)xanthene)-3-one, 3'6'-dihydroxy, Disodium Salt
22. Funduscein
23. Flurenate
24. Furanium
25. Obiturine
26. Hidacid Uranine
27. Uranine Yellow
28. Fluorescein Lt
29. Fluoresein Sodium
30. Uranine A
31. Uranine O
32. Ak-fluor
33. Uranin A
34. Uranin S
35. Uranine A Extra
36. Uranine Ss
37. Fluor-i-strip
38. Yellow 8
39. Soluble Fluoresceine
40. Floures (tn)
41. Sodium Fluoresceinate
42. Certiqual Fluoresceine
43. Fluoresceinum Natricum
44. Fluorescein Free Acid
45. Basacid Yellow 226
46. Fluor-i-strip A.t.
47. Fluorescein Sodium B.p
48. D&c Yellow No. 8
49. Fluorescein, Sodium Salt
50. 11824 Yellow
51. 12417 Yellow
52. Soluble Fluoresceine Bps
53. Japan Yellow 202(1)
54. D & C Yellow No. 8
55. Acid Yellow 73 Sodium Salt
56. Schembl264060
57. Unii-93x55pe38x
58. Ci 45350 (na Salt)
59. Ccris 6239
60. Chembl1628233
61. Fluorescein Sodium [jan]
62. Fluorescein Sodium (jp17/usp)
63. Hsdb 8007
64. Fluorescein Sodium [vandf]
65. Ki202(1) [inci]
66. Ki202(2) [inci]
67. Moli001004
68. Nci-c54706
69. Fluorescein Sodium [mart.]
70. Fluorescein Sodium [usp:ban:jan]
71. Fluorescein Sodium [who-dd]
72. Fluorescein Sodium [who-ip]
73. Nsc 5070
74. Einecs 208-253-0
75. Akos024364966
76. Fluorescein Disodium Salt [mi]
77. Fluorescein Sodium [ep Impurity]
78. Fluorescein Sodium [orange Book]
79. Fluorescein Sodium [ep Monograph]
80. As-17142
81. Fluorescein Sodium [usp Monograph]
82. F0096
83. Fluoresceinum Natricum [who-ip Latin]
84. Ft-0626450
85. D02024
86. Disodium 6-hydroxy-3-oxo-9-xanthene-o-benzoate
87. Sodium Salt Of Hydroxy-o-carboxy-phenyl-fluorone
88. Fluorescein Sodium Component Of Altafluor Benox
89. Q27271622
90. Z1262246141
91. 9-o-carboxyphenyl-6-hydroxy-3-isoxanthone, Disodium Salt
92. Benzoic Acid, 2-(6-hydroxy-3-oxo-3h-xanthen-9-yl)-, Disodium Salt
93. 3',6'-dihydroxyspiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one, Disodium Salt
94. Spiro(isobenzofuran-1(3h),9'-(9h)xanthen)-3-one, 3',6'-dihydroxy-, Disodium Salt
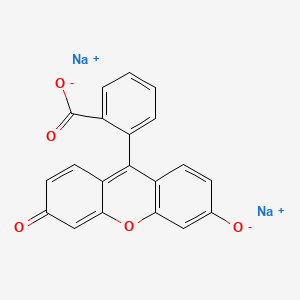
| Molecular Weight | 376.3 g/mol |
|---|---|
| Molecular Formula | C20H10Na2O5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 376.03236198 g/mol |
| Monoisotopic Mass | 376.03236198 g/mol |
| Topological Polar Surface Area | 89.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 644 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Contrast Media; Fluorescent Dyes
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
/Fluorescein sodium/ 10% (100 mg/mL) and 25% (250 mg/mL) is indicated in diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
Fluorescein injection, (USP) 10% is indicated in diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature.
US Natl Inst Health; DailyMed. Current Medication Information for Fluorescite (fluorescein sodium) injection, solution (2009). Available from, as of October 19, 2009: https://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=Fluorescein+sodium
Uranine is used topically in the fitting of hard contact lenses and in other ocular diagnostic and therapeutic areas, particularly in corneal challenges.
The National Academies; Institute of Medicine: Health Effects of Project Shad Chemical Agent: Uranine Dye (Sodium Fluorescein) 518-47-8 p. 5 (2004). Available from, as of October 24, 2011: https://www.iom.edu/Reports.aspx
For more Therapeutic Uses (Complete) data for D&C Yellow No. 8 (9 total), please visit the HSDB record page.
/Fluorescein sodium/ is contraindicated in patients with known hypersensitivity to fluorescein sodium or any other ingredients in this product. Rare cases of death due to anaphylaxis have been reported.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
Caution should be exercised in patients with a history of allergy or bronchial asthma. An emergency tray should always be available.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
If a potential allergy is suspected, an intradermal skin test may be performed prior to intravenous administration, i.e., 0.05 mL injected intradermally to be evaluated 30 to 60 minutes following injection. Given the sensitivity and specificity of skin testing, a negative skin test is not proof that a patient is not allergic to fluorescein.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
Extravasation during injection can result in severe local tissue damage due to high pH of fluorescein solution. The following complications resulting from extravasation of fluorescein have been noted to occur: Sloughing of the skin, superficial phlebitis, subcutaneous granuloma, and toxic neuritis along the median nerve in the antecubital area. Complications resulting from extravasation can cause severe pain in the arm for up to several hours. When extravasation occurs, the injection should be discontinued and conservative measures to treat damaged tissue and to relieve pain should be implemented.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
For more Drug Warnings (Complete) data for D&C Yellow No. 8 (13 total), please visit the HSDB record page.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Fluorescent Dyes
Chemicals that emit light after excitation by light. The wave length of the emitted light is usually longer than that of the incident light. Fluorochromes are substances that cause fluorescence in other substances, i.e., dyes used to mark or label other compounds with fluorescent tags. (See all compounds classified as Fluorescent Dyes.)
Uranine is rapidly absorbed in the lungs and in the respiratory tract, and is affected by the presence of surfactants. It is not metabolized in the lung. Inhaled uranine is excreted in the urine.
The National Academies; Institute of Medicine: Health Effects of Project Shad Chemical Agent: Uranine Dye (Sodium Fluorescein) 518-47-8 p. 9 (2004). Available from, as of October 24, 2011: https://www.iom.edu/Reports.aspx
Within 7 to 14 seconds after IV administration into the antecubital vein, fluorescein usually appears in the central retinal artery of the eye. Within a few minutes of IV administration of fluorescein sodium, a yellowish discoloration of the skin occurs, which begins to fade 6 to 12 hours after dosing. Various estimates of volume of distribution indicate that fluorescein distributes into interstitial space (0.5 L/kg).
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
Fluorescein sodium has been demonstrated to be excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
Fluorescein and its metabolite are mainly eliminated via renal excretion. After IV administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 mL/min/kg and a hepatic clearance (due to conjugation) of 1.50 mL/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
For more Absorption, Distribution and Excretion (Complete) data for D&C Yellow No. 8 (7 total), please visit the HSDB record page.
Fluorescein is metabolized to fluorescein monoglucuronide. After IV administration of fluorescein sodium (14 mg/kg) to 7 healthy subjects, approximately 80% of fluorescein in plasma was converted to glucuronide conjugate after a period of 1 hour post dose.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
... The pharmacokinetics of fluorescein and fluorescein glucuronide /were studied/ for 38 hr in the plasma of five normal subjects given 14 mg/kg of sodium fluorescein intravenously. ... The terminal half-lives of fluorescein and fluorescein glucuronide in the plasma ultrafiltrate were 23.5 and 264 min, respectively, ... .
PMID:3721789 Blair NP et al; Invest Ophthalmol Vis Sci 27 (7): 1107-14 (1986).
Fluorescein sodium responds to electromagnetic radiation and light between the wavelengths of 465 to 490 nm and fluoresces, i.e., emits light at wavelengths of 520 to 530 nm. Thus, the hydrocarbon is excited by blue light and emits light that appears yellowish green. Following intravenous injection of fluorescein sodium in an aqueous solution, the unbound fraction of the fluorescein can be excited with a blue light flash from a fundus camera as it circulates through the ocular vasculature, and the yellowish green fluorescence of the dye is captured by the camera. In the fundus, the fluorescence of the dye demarcates the retinal and/or choroidal vasculature under observation, distinguishing it from adjacent areas/structures.
US Natl Inst Health; DailyMed. Current Medication Information for AK-FLUOR (fluorescein sodium) injection (August 2011). Available from, as of October 21, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a1823be3-a739-48bc-a3ea-ceb7cc5105ce
ABOUT THIS PAGE
19
PharmaCompass offers a list of Fluorescein Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluorescein Sodium manufacturer or Fluorescein Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluorescein Sodium manufacturer or Fluorescein Sodium supplier.
PharmaCompass also assists you with knowing the Fluorescein Sodium API Price utilized in the formulation of products. Fluorescein Sodium API Price is not always fixed or binding as the Fluorescein Sodium Price is obtained through a variety of data sources. The Fluorescein Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aizen Uranine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aizen Uranine, including repackagers and relabelers. The FDA regulates Aizen Uranine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aizen Uranine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aizen Uranine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aizen Uranine supplier is an individual or a company that provides Aizen Uranine active pharmaceutical ingredient (API) or Aizen Uranine finished formulations upon request. The Aizen Uranine suppliers may include Aizen Uranine API manufacturers, exporters, distributors and traders.
click here to find a list of Aizen Uranine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aizen Uranine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aizen Uranine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aizen Uranine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aizen Uranine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aizen Uranine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aizen Uranine suppliers with NDC on PharmaCompass.
Aizen Uranine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aizen Uranine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aizen Uranine GMP manufacturer or Aizen Uranine GMP API supplier for your needs.
A Aizen Uranine CoA (Certificate of Analysis) is a formal document that attests to Aizen Uranine's compliance with Aizen Uranine specifications and serves as a tool for batch-level quality control.
Aizen Uranine CoA mostly includes findings from lab analyses of a specific batch. For each Aizen Uranine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aizen Uranine may be tested according to a variety of international standards, such as European Pharmacopoeia (Aizen Uranine EP), Aizen Uranine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aizen Uranine USP).
