
Synopsis
Synopsis
0
VMF
0
Australia

Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
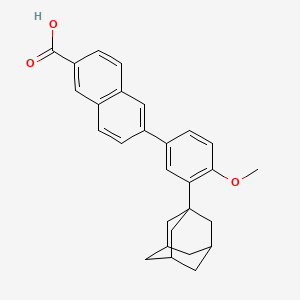

1. 271, Cd
2. 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic Acid
3. Adaferin
4. Cd 271
5. Cd-271
6. Cd271
7. Differin
8. Differine
1. 106685-40-9
2. Differin
3. Adapaleno
4. 6-(3-(adamantan-1-yl)-4-methoxyphenyl)-2-naphthoic Acid
5. Adapalenum
6. Adapalenum [inn-latin]
7. Adapaleno [inn-spanish]
8. Cd 271
9. Cd-271
10. 6-[3-(1-adamantyl)-4-methoxyphenyl]naphthalene-2-carboxylic Acid
11. 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic Acid
12. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic Acid
13. Mfcd03106112
14. 6-[3-(1-adamantyl)-4-methoxy-phenyl]naphthalene-2-carboxylic Acid
15. Chembl1265
16. 6-(4-methoxy-3-tricyclo[3.3.1.13,7]dec-1-ylphenyl)-2-naphthalenecarboxylic Acid
17. Adaferin
18. Chebi:31174
19. 1l4806j2qf
20. 6-(3-adamantan-1-yl-4-methoxyphenyl)naphthalene-2-carboxylic Acid
21. Ncgc00164617-01
22. Adapalen
23. Differine
24. 6-[3-(adamantan-1-yl)-4-methoxyphenyl]naphthalene-2-carboxylic Acid
25. 2-naphthalenecarboxylic Acid, 6-(4-methoxy-3-tricyclo(3.3.1.1(sup 3,7))dec-1-ylphenyl)-
26. Dsstox_cid_26481
27. Dsstox_rid_81652
28. Dsstox_gsid_46481
29. Differin Gel
30. Cd271
31. Differin (tn)
32. Cas-106685-40-9
33. Differin Gel 0.1%
34. Adapalene [usan:inn:ban]
35. Unii-1l4806j2qf
36. Adapalene- Bio-x
37. Ks-1196
38. Adapalene [inn]
39. Adapalene [jan]
40. Adapalene [mi]
41. Adapalene [usan]
42. Adapalene [vandf]
43. Adapalene [mart.]
44. Adapalene [usp-rs]
45. Adapalene [who-dd]
46. Schembl2747
47. Adapalene (jan/usp/inn)
48. Mls000759463
49. Mls006010036
50. Bidd:gt0264
51. Idp-126 Component Adapalene
52. Gtpl5429
53. Adapalene [orange Book]
54. Adapalene [ep Monograph]
55. Adapalene, >=98% (hplc)
56. Chembl4303650
57. Dtxsid5046481
58. Epiduo Component Adapalene
59. Adapalene [usp Monograph]
60. Hms3264f15
61. Hms3654f11
62. Hms3715h16
63. Bcp02081
64. Hy-b0091
65. Zinc3784182
66. Adapalene Component Of Epiduo
67. Tox21_112236
68. Bdbm50048280
69. S1276
70. Stl453114
71. Akos005145841
72. Akos015895391
73. Tox21_112236_1
74. Ab13763
75. Ac-1974
76. Bcp9000231
77. Ccg-213060
78. Ccg-221237
79. Cs-1789
80. Db00210
81. Ncgc00164617-02
82. Ncgc00164617-04
83. Ncgc00164617-05
84. Ba164138
85. Smr000466349
86. Smr002529673
87. Sy009767
88. A2549
89. Ft-0631040
90. Sw219282-1
91. D01112
92. Ab01274764-01
93. Ab01274764-02
94. Ab01274764_03
95. Ab01274764_04
96. 685a409
97. A801483
98. Q352348
99. Sr-01000942194
100. Sr-01000942194-2
101. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoicacid
102. Brd-k33127281-001-01-5
103. F2173-0588
104. 6-(3-(adamantan-1-yl)-4-methoxyphenyl)-2-naphthoicacid
105. Adapalene 100 Microg/ml In Acetonitrile:dimethylsulfoxide
106. Adapalene, European Pharmacopoeia (ep) Reference Standard
107. Adapalene, United States Pharmacopeia (usp) Reference Standard
108. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthalene-carboxylic Acid
109. 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthalenecarboxylic Acid
110. 6-(3-((3r,5r,7r)-adamantan-1-yl)-4-methoxyphenyl)-2-naphthoic Acid
111. Adapalene, Pharmaceutical Secondary Standard; Certified Reference Material
112. 6-[3-(1-adamantyl)-4-methoxy-phenyl]naphthalene-2-carboxylic Acid;adapalene
113. 6-[4-methoxy-3-(tricyclo[3.3.1.1~3,7~]dec-1-yl)phenyl]naphthalene-2-carboxylic Acid
114. 6-[4-methoxy-3-(tricyclo[3.3.1.13,7]dec-1-yl)phenyl]naphthalene-2-carboxylic Acid
115. Adapalene For Peak Identification, European Pharmacopoeia (ep) Reference Standard
116. 2-naphthalenecarboxylic Acid, 6-(4-methoxy-3-tricyclo(3.3.1.(sup 13,7))dec-1-ylphenyl)-
| Molecular Weight | 412.5 g/mol |
|---|---|
| Molecular Formula | C28H28O3 |
| XLogP3 | 7.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 412.20384475 g/mol |
| Monoisotopic Mass | 412.20384475 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Adapalene |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | Adapalene Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of Adapalene Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer homopolymer type C, disodium edetate, methylparaben, poloxamer 182,... |
| Active Ingredient | Adapalene |
| Dosage Form | Gel; Cream |
| Route | topical; Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Glenmark Generics; Fougera Pharms; Actavis Mid Atlantic; Pliva Hrvatska Doo; Tolmar |
| 2 of 4 | |
|---|---|
| Drug Name | Differin |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | DIFFERIN Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of DIFFERIN Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glyc... |
| Active Ingredient | Adapalene |
| Dosage Form | Lotion; Gel; Cream |
| Route | Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Adapalene |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | Adapalene Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of Adapalene Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer homopolymer type C, disodium edetate, methylparaben, poloxamer 182,... |
| Active Ingredient | Adapalene |
| Dosage Form | Gel; Cream |
| Route | topical; Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Tentative Approval; Prescription |
| Company | Glenmark Generics; Fougera Pharms; Actavis Mid Atlantic; Pliva Hrvatska Doo; Tolmar |
| 4 of 4 | |
|---|---|
| Drug Name | Differin |
| PubMed Health | Adapalene (On the skin) |
| Drug Classes | Antiacne |
| Drug Label | DIFFERIN Gel, containing adapalene, is used for the topical treatment of acne vulgaris. Each gram of DIFFERIN Gel contains adapalene 0.1% (1 mg) in a vehicle consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 182, propylene glyc... |
| Active Ingredient | Adapalene |
| Dosage Form | Lotion; Gel; Cream |
| Route | Topical |
| Strength | 0.3%; 0.1% |
| Market Status | Prescription |
| Company | Galderma Labs |
Adapalene is indicated for the topical treatment of acne vulgaris in patients aged 12 and over.
FDA Label
Adapalene is anticomedogenic, preventing the formation of new comedones and inflammatory lesions, and also acts to reduce inflammation by modulating the innate immune response. Like other retinoid compounds, adapalene is chemically stable but photosensitive; use with sunscreen is recommended. Minor skin irritations, including erythema, scaling, dryness, and stinging/burning, have been reported.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D10AD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AD - Retinoids for topical use in acne
D10AD03 - Adapalene
Absorption
Adapalene is applied topically and absorbed through the skin. In one clinical study treating patients once per day with 2g of 0.3% gel applied to 2 mg/cm2 of skin, 15 patients had detectable blood plasma adapalene levels (0.1 ng/ml) resulting in a mean Cmax of 0.553 0.466 ng/ml and a mean AUC of 8.37 8.46 ng\*h/ml on day 10.
Route of Elimination
Adapalene is primarily excreted by the biliary route at about 30 ng/g of the topically applied amount. Approximately 75% of the drug remains unchanged.
Clearance
Adapalene is rapidly cleared from blood plasma, typically undetectable after 72 hours following topical application.
Extensive information regarding adapalene metabolism in humans is unavailable, although it is known to accumulate in the liver and GI-tract. In human, mouse, rat, rabbit, and dog cultured hepatocytes, metabolism appears to affect the methoxybenzene moiety but remains incompletely characterized. The major products of metabolism are glucuronides. Approximately 25% of the drug is metabolized; the rest is excreted as parent drug.
In one clinical study, after ten days of treatment with 2g of 0.3% cream or gel, the terminal half-life was between 7 and 51 hours, with a mean of 17.2 10.2.
Adapalene is used for the treatment/maintenance of mild-to-severe acne (acne vulgaris). Acne is a multifactorial condition, and evidence exists to support multiple mechanisms of action for adapalene. Adapalene binds to retinoic acid receptor (RAR)-beta and RAR-gamma; this complex subsequently binds to one of three retinoid X receptors (RXRs), which as a complex is capable of binding DNA to modulate transcriptional activity. Although the full extent of transcriptional modulation is not described, retinoid activation is generally known to affect cellular proliferation and differentiation, and adapalene has been shown to inhibit HeLa cell proliferation and human keratinocyte differentiation. These effects primarily account for adapalene's comedolytic and anticomedogenic properties. In addition, adapalene modulates the immune response by down-regulating toll-like receptor 2 (TLR-2) expression and inhibiting the transcription factor activator protein 1 (AP-1). TLR-2 recognizes _Cutibacterium acnes_ (formerly _Propionibacterium acnes_), the bacterium primarily associated with acne. TLR-2 activation causes nuclear translocation of AP-1 and downstream pro-inflammatory gene regulation. Therefore, adapalene has a general anti-inflammatory effect, which reduces inflammation-mediated acne symptoms. When used with benzoyl peroxide, which possesses free radical-mediated bactericidal effects, the combination acts synergistically to reduced comedones and inflammatory lesions.

Certificate Number : R1-CEP 2016-309 - Rev 00
Issue Date : 2023-03-02
Type : Chemical
Substance Number : 2445
Status : Valid

Registration Number : 228MF10176
Registrant's Address : 5th Floor, Lakshminarayan Complex, 10/1, Palace Road, Bangalore-560052, India
Initial Date of Registration : 2016-08-29
Latest Date of Registration :

Date of Issue : 2022-08-25
Valid Till : 2025-07-02
Written Confirmation Number : WC-0156
Address of the Firm :

Registrant Name : Galderma Korea Co., Ltd.
Registration Date : 2024-03-27
Registration Number : 20240327-209-J-1630
Manufacturer Name : FINORGA SAS
Manufacturer Address : Route de Givors Chasse Sur Rhone,38670, France
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-05-30
Pay. Date : 2013-05-21
DMF Number : 22709
Submission : 2009-04-08
Status : Active
Type : II

Certificate Number : CEP 2022-158 - Rev 00
Issue Date : 2024-06-19
Type : Chemical
Substance Number : 2445
Status : Valid

Registration Number : 228MF10126
Registrant's Address : 71, UDYOG KSHETRA, ULND-GOREGAON LINK ROAD, MULUND (W), MUMBAI 400080, INDIA
Initial Date of Registration : 2016-07-13
Latest Date of Registration :

Date of Issue : 2023-01-06
Valid Till : 2025-07-29
Written Confirmation Number : WC-0099
Address of the Firm :

NDC Package Code : 15308-0716
Start Marketing Date : 1997-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2023-09-13
Registration Number : 20230110-209-J-1433(1)
Manufacturer Name : Aarti Pharmalabs limited
Manufacturer Address : Unit – IV, Plot No: E – 50, 50/1 & 59/1, MIDC, Tarapur, Taluka & District: Palghar, Pin-401 506 Maharashtra, India.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.

Registrant Name : Galderma Korea Co., Ltd.
Registration Date : 2023-08-24
Registration Number : 20230824-209-J-1526
Manufacturer Name : FINORGA SAS
Manufacturer Address : route de Givors Chasse Sur Rhone,38670, France
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.

Registrant Name : Iksoo Pharmaceutical Co., Ltd.
Registration Date : 2023-01-10
Registration Number : 20230110-209-J-1433
Manufacturer Name : Aarti Pharmalabs limited
Manufacturer Address : Unit – IV, Plot No: E – 50, 50/1 & 59/1, MIDC, Tarapur, Taluka & District: Palghar, Pin-401 506 Maharashtra, India.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18831
Submission : 2005-09-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
46
PharmaCompass offers a list of Adapalene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Adapalene manufacturer or Adapalene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Adapalene manufacturer or Adapalene supplier.
PharmaCompass also assists you with knowing the Adapalene API Price utilized in the formulation of products. Adapalene API Price is not always fixed or binding as the Adapalene Price is obtained through a variety of data sources. The Adapalene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Adapalene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Adapalene, including repackagers and relabelers. The FDA regulates Adapalene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Adapalene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Adapalene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Adapalene supplier is an individual or a company that provides Adapalene active pharmaceutical ingredient (API) or Adapalene finished formulations upon request. The Adapalene suppliers may include Adapalene API manufacturers, exporters, distributors and traders.
click here to find a list of Adapalene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Adapalene DMF (Drug Master File) is a document detailing the whole manufacturing process of Adapalene active pharmaceutical ingredient (API) in detail. Different forms of Adapalene DMFs exist exist since differing nations have different regulations, such as Adapalene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Adapalene DMF submitted to regulatory agencies in the US is known as a USDMF. Adapalene USDMF includes data on Adapalene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Adapalene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Adapalene suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Adapalene Drug Master File in Japan (Adapalene JDMF) empowers Adapalene API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Adapalene JDMF during the approval evaluation for pharmaceutical products. At the time of Adapalene JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Adapalene suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Adapalene Drug Master File in Korea (Adapalene KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Adapalene. The MFDS reviews the Adapalene KDMF as part of the drug registration process and uses the information provided in the Adapalene KDMF to evaluate the safety and efficacy of the drug.
After submitting a Adapalene KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Adapalene API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Adapalene suppliers with KDMF on PharmaCompass.
A Adapalene CEP of the European Pharmacopoeia monograph is often referred to as a Adapalene Certificate of Suitability (COS). The purpose of a Adapalene CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Adapalene EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Adapalene to their clients by showing that a Adapalene CEP has been issued for it. The manufacturer submits a Adapalene CEP (COS) as part of the market authorization procedure, and it takes on the role of a Adapalene CEP holder for the record. Additionally, the data presented in the Adapalene CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Adapalene DMF.
A Adapalene CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Adapalene CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Adapalene suppliers with CEP (COS) on PharmaCompass.
A Adapalene written confirmation (Adapalene WC) is an official document issued by a regulatory agency to a Adapalene manufacturer, verifying that the manufacturing facility of a Adapalene active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Adapalene APIs or Adapalene finished pharmaceutical products to another nation, regulatory agencies frequently require a Adapalene WC (written confirmation) as part of the regulatory process.
click here to find a list of Adapalene suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Adapalene as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Adapalene API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Adapalene as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Adapalene and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Adapalene NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Adapalene suppliers with NDC on PharmaCompass.
Adapalene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Adapalene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Adapalene GMP manufacturer or Adapalene GMP API supplier for your needs.
A Adapalene CoA (Certificate of Analysis) is a formal document that attests to Adapalene's compliance with Adapalene specifications and serves as a tool for batch-level quality control.
Adapalene CoA mostly includes findings from lab analyses of a specific batch. For each Adapalene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Adapalene may be tested according to a variety of international standards, such as European Pharmacopoeia (Adapalene EP), Adapalene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Adapalene USP).
