Top news of 2025: Drugmakers invest in US capacities, agree to lower Medicaid prices; Pfizer buys obesity-focused biotech Metsera
The year 2025 was an eventful one, marked by increased trade
tensions, tariff threats, accelerated
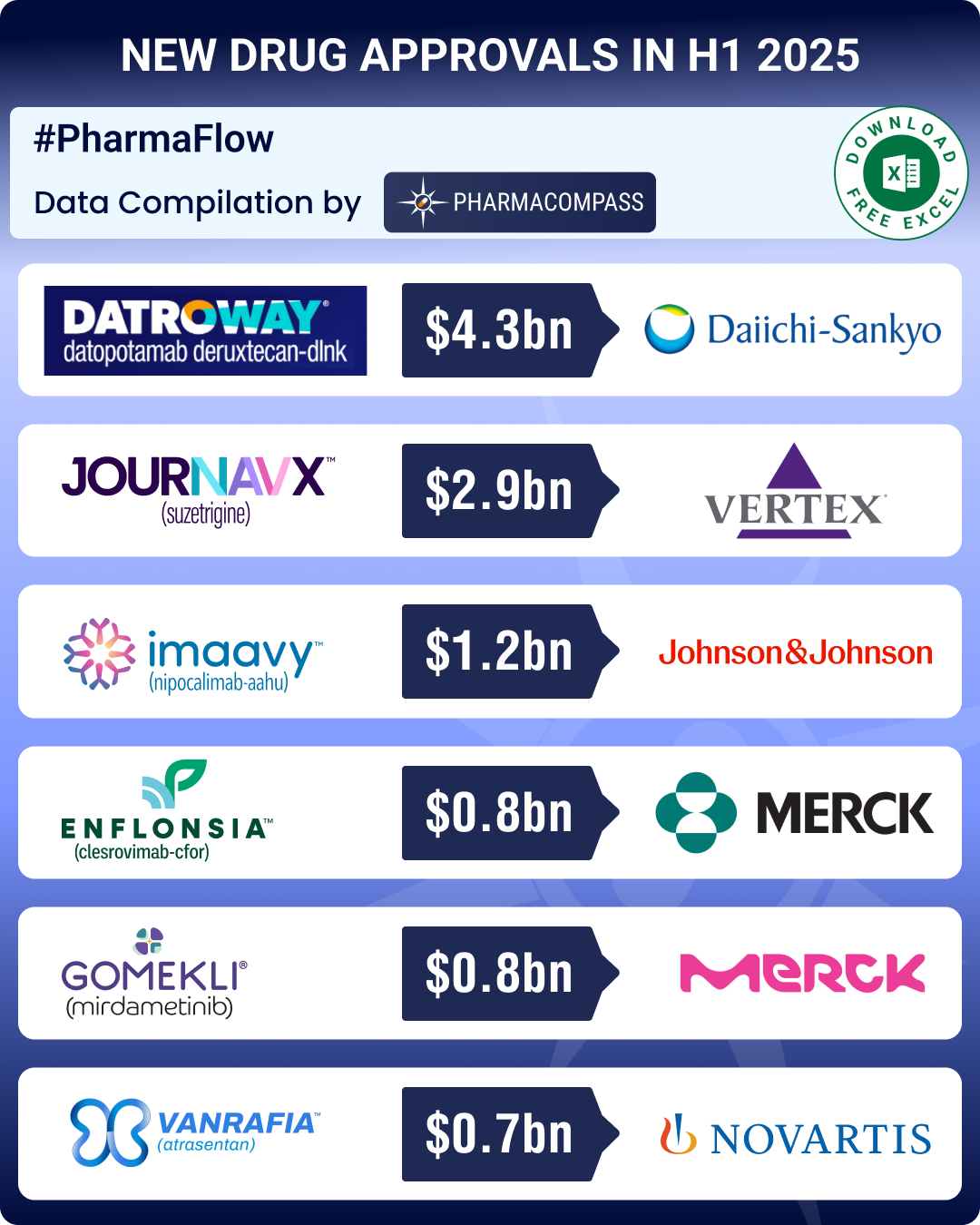
FDA approvals drop 24% in H1 2025; GSK’s UTI med, Vertex’s non-opioid painkiller lead pack of first-in-class meds
It has been a turbulent year for the US
Food and Drug Administration (FDA), marked by reduction
J&J’s Intra‑Cellular buyout, BMS’ oncology gambit, Sanofi’s Blueprint acquisition drive mega deals in H1 2025
The pharmaceutical industry has witnessed a wave of mergers, acquisitions, and strategic partnership
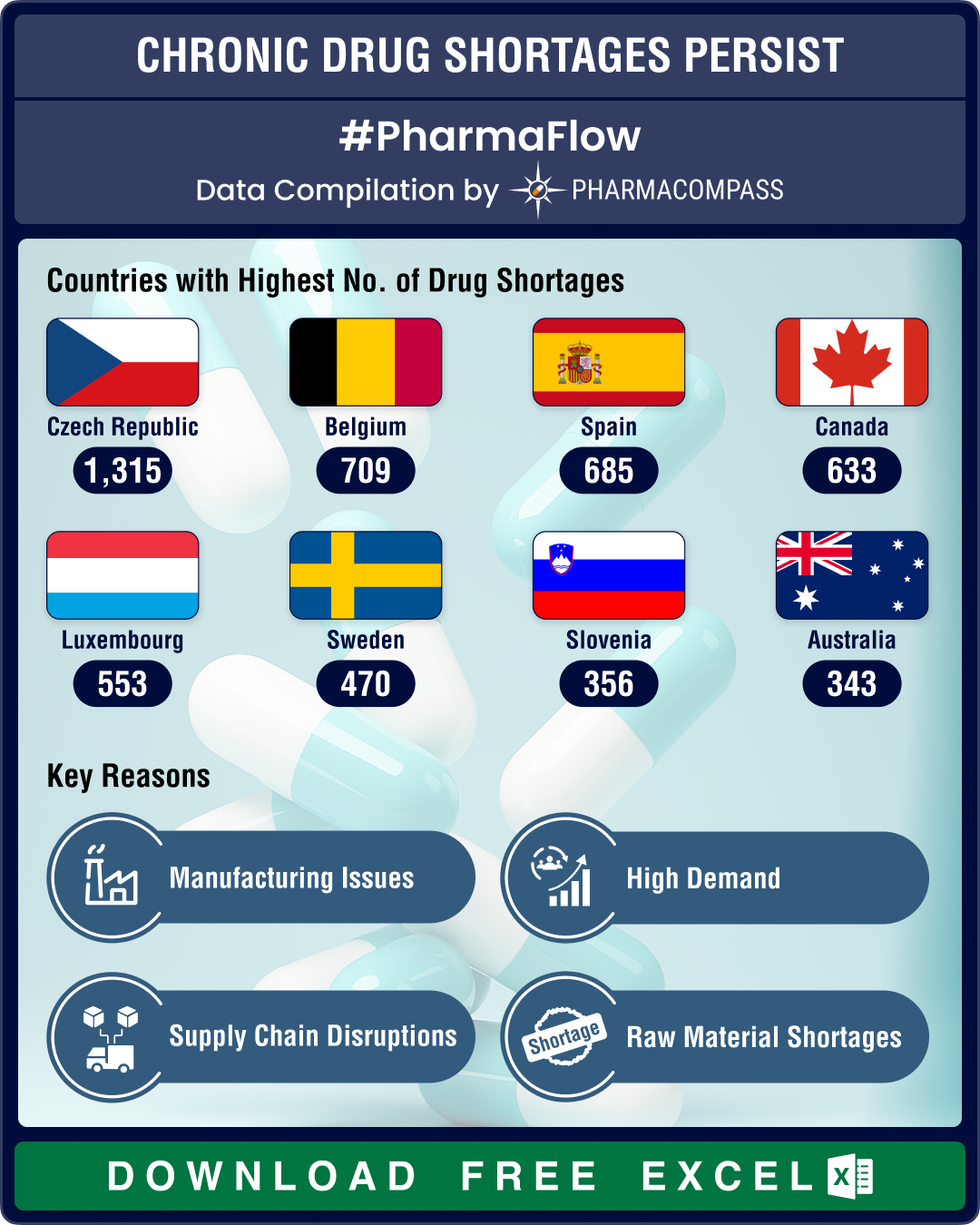
US drug shortages reduce 16% YoY in Q1 2025; CNS drugs, antimicrobials face highest scarcities
The pharmaceutical industry in the United States continued to
grapple with drug shortages during th
Top Pharma Companies & Drugs in 2024: Merck’s Keytruda maintains top spot as Novo’s semaglutide nips at its heels
In 2024, Big Pharma players consolidated
and maintained their dominance, even as innovation continu
Top first-in-class drug candidates of 2025: Ionis’ donidalorsen, Sanofi’s fitusiran, Cytokinetics’ aficamten await FDA approval
First‑in‑class drugs are therapies with entirely new approaches that improve patient out
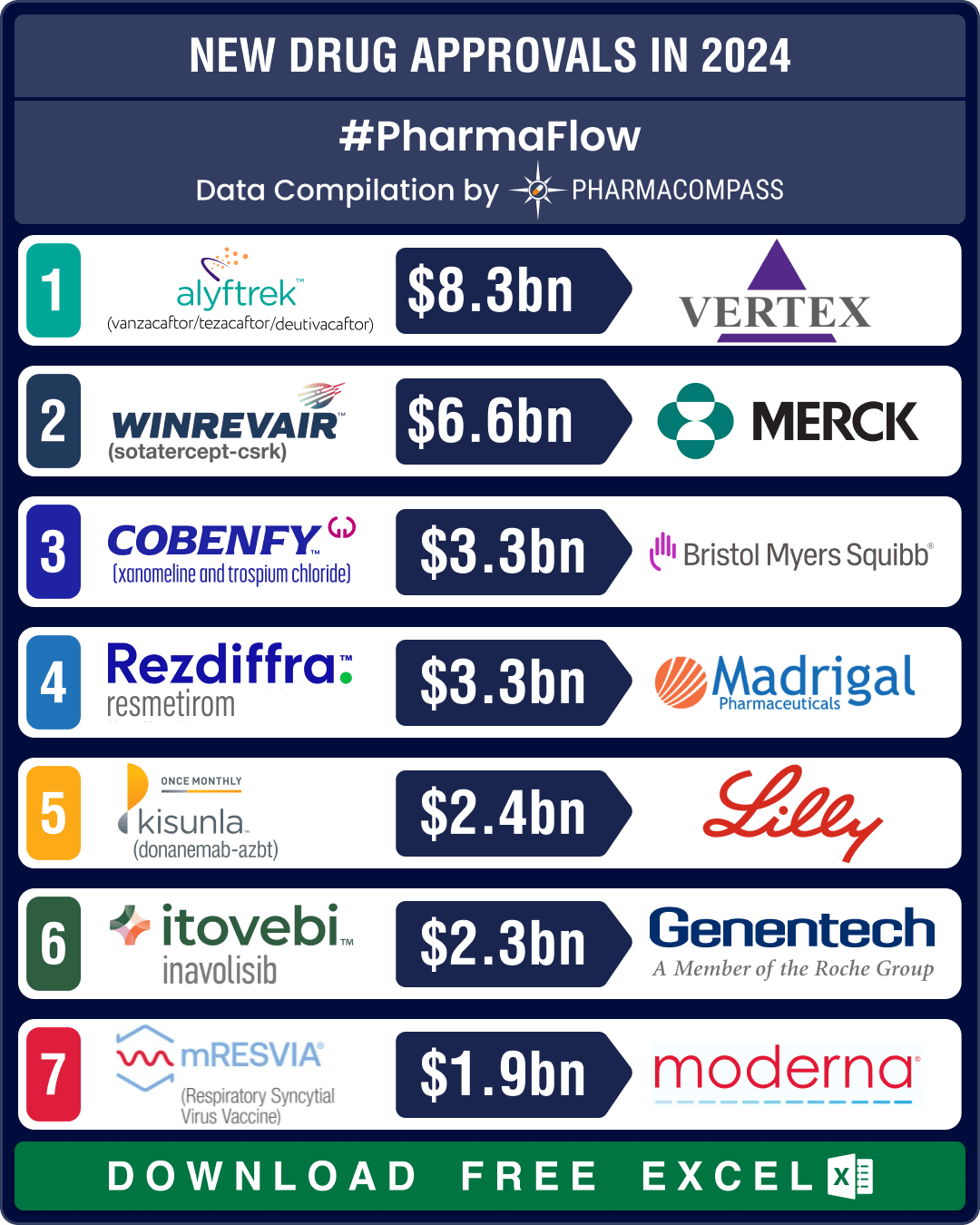
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
BMS, J&J, Bayer lead 25,000+ pharma layoffs in 2024; Amylyx, FibroGen, Kronos Bio hit by trial failures, cash crunch
Since 2022, there has been a significant surge in layoffs by pharmaceutical and biotech companies. W
FDA’s landmark approvals of BMS’ schizo med, Madrigal’s MASH drug, US$ 16.5 bn Catalent buyout make it to top 10 news of 2024
The year 2024 was marked by some landmark drug approvals in the areas of schizophrenia, metabolic dy


 Market Place
Market Place Sourcing Support
Sourcing Support