By PharmaCompass
2024-01-18
Impressions: 3188
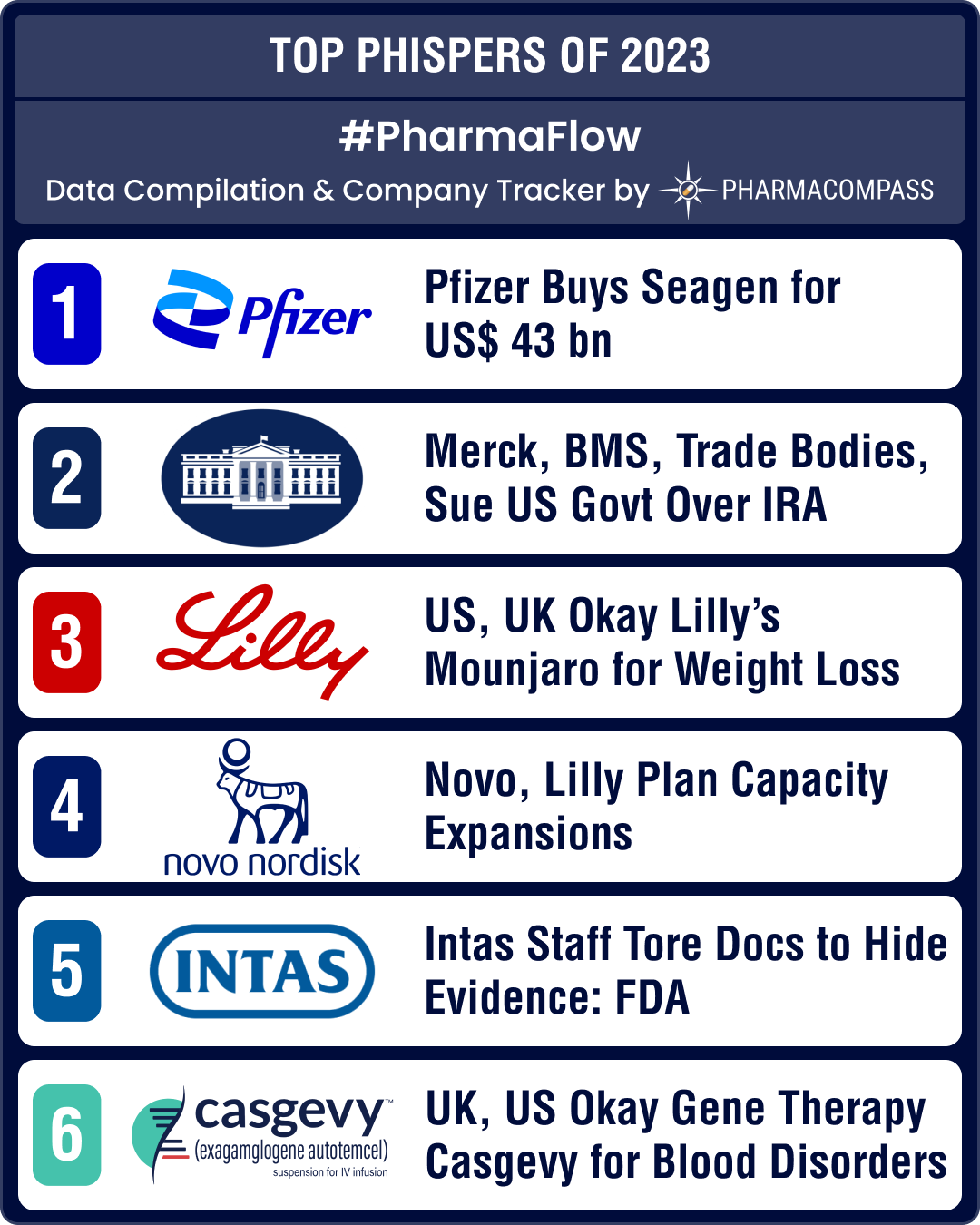
Every week, PharmaCompass compiles important developments in the world of pharmaceuticals and brings a compilation to you in the form of Phispers. Of the hundreds of stories we carried in 2023, here are the top 10 stories, including some trends and updates.
I. Pfizer buys Seagen for US$ 43 billion to bolster its oncology portfolio
In March, Pfizer said it is acquiring cancer treatment specialist Seagen for US$ 43 billion. Seagen is a pioneer in antibody-drug conjugates (ADCs), or drugs that work like “guided missiles” to destroy cancer cells while sparing healthy cells. Another important deal in the field of ADCs took place in December when AbbVie picked up ImmunoGen for US$ 10.1 billion, giving it access to Elahere (mirvetuximab soravtansine-gynx), an ADC approved for platinum-resistant ovarian cancer. Elahere is expected to achieve blockbuster status by 2030.
II. Merck, BMS, trade bodies, sue US government over IRA negotiations
In June, Merck filed a lawsuit against the US government seeking to block Medicare from negotiating lower prescription drug prices under the Inflation Reduction Act (IRA). Days later, the US Chamber of Commerce, one of the most influential trade groups in the US, filed a separate lawsuit, arguing that the negotiations violated drugmakers’ constitutional rights and granted excessive control over prices to the government. They were joined by Bristol Myers Squibb (BMS) and lobby group PhRMA.
Drugmakers and the Biden administration appeared to be at each other’s throats. In December, the White House identified 48 drugs whose prices spiked faster than inflation in Q4. These drugs may be subject to rebates starting January 2024. Biden Administration also announced it is setting a new “march-in” policy that allows the government to seize medicine patents held by drugmakers for therapies whose development was taxpayer-funded, if it believes they are not “reasonably available and affordable.”
III. US, UK approve Lilly’s Mounjaro for weight management; to be sold as Zepbound
In November, drug regulators in the US and the United Kingdom approved Eli Lilly's Mounjaro (tirzepatide) for weight management, to be sold under the brand name Zepbound. The drug will pose strong competition to Novo Nordisk’s Wegovy in a market that's expected to reach US$ 100 billion by the end of the decade.
IV. Novo, Lilly plan capacity expansions for weight loss drugs
Both Novo Nordisk and Eli Lilly announced expanding their manufacturing capacities in order to capitalize on the burgeoning market for weight loss drugs. Novo is investing over DKK 42 billion (US$ 6 billion) in Kalundborg (Denmark), US$ 2.3 billion to expand its site in Chartres (France) and over € 2 billion (US$ 2.18 billion) in Dublin (Ireland) to boost production of its blockbuster diabetes and weight-loss drugs, including Ozempic and Wegovy (both semaglutide). Similarly, Eli Lilly had announced a US$ 2.5 billion manufacturing facility in Germany in November to address the demand for its new obesity and diabetes therapies.
V. FDA finds violations at Global Pharma’s eye drops plant in India; issues Form 483
In April, FDA found several violations in manufacturing processes and sterilization methods used by India-based Global Pharma for its EzriCare Artificial Tears Eye Drop, which has been linked to 68 cases of eye infection in the US, including eight cases of vision loss and three deaths.
VI. ‘Intas India staff tore documents, threw acid to destroy evidence’, notes FDA
In January, FDA issued a Form 483 with 11 observations to Intas Pharma’s drug manufacturing facility in Ahmedabad (Gujarat, India). A team of three FDA drug regulators conducted an inspection of the manufacturing facility from November 22 to December 2, 2022. The 36-page report issued by the FDA has alleged that employees at the facility had destroyed documents related to manufacturing practices by tearing them into pieces and disposing them inside the quality control lab and scrap areas. Acid was used to destroy evidence, notes FDA.
VII. GSK overtakes Pfizer in bagging first FDA approval for RSV vaccine
In May, FDA approved GSK’s respiratory syncytial virus (RSV) vaccine for people aged 60 and above. Arexvy is the first RSV vaccine to be approved in the US for the common condition that can be fatal for the elderly. Later that month, Pfizer’s RSV vaccine Abrysvo also got approved. In July, Sanofi-AstraZeneca’s RSV antibody therapy, Beyfortus (nirsevimab-alip), received approval from the FDA. It is a long-acting treatment that can be given once per season. The approval is specifically developed for newborns and infants.
VIII. UK authorizes gene therapy Casgevy for blood disorders, US follows suit
In November, Britain’s Medicines and Healthcare products Regulatory Agency was first off the block in authorizing CRISPR Therapeutics and Vertex Pharmaceuticals’ Casgevy, a therapy that seeks to cure two blood disorders — sickle-cell disease (SCD) and β-thalassemia. The therapy is based on gene editing technology that had won its scientists the Nobel Prize in Chemistry in 2020.Less than a month later, FDA not only approved Casgevy (exagamglogene autotemcel) for SCD, but also approved bluebird bio’s Lyfgenia (lovotibeglogene autotemcel) for the treatment of SCD in patients aged 12 and older who have a history of vaso-occlusive events (when tissues become deprived of oxygen).
IX. Leqembi becomes first med to bag full approval to treat Alzheimer’s
Eisai and Biogen’s Alzheimer’s drug Leqembi (lecanemab) had won FDA’s accelerated approval in January. It treats patients who are in the earliest stages of the neurodegenerative disease. In July, it became the first treatment to receive full FDA approval to treat the condition.
X. Bayer’s experimental anticoagulant fails late-stage trial
One of the biggest disappointments from clinical trials came when a major late-stage trial for Bayer’s experimental anticoagulant asundexian had to be discontinued due to its inadequate effectiveness. Bayer had expectations in excess of € 5 billion (US$ 5.5 billion) from this drug.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Top Phispers of 2023 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”






