By PharmaCompass
2021-07-22
Impressions: 3740
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan that is found in almost all connective tissues in mammals. It is found in the highest concentrations in fluids in the eyes and joints.
Sodium hyaluronate is the salt form of hyaluronic acid. Both HA and sodium
hyaluronate are counterparts of each other, offering the same kind
of benefits.
Hyaluronic acid, used in drugs, is extracted from rooster combs or made by bacteria in laboratories. It has various therapeutic applications and has become an essential ingredient in beauty and skincare products today.
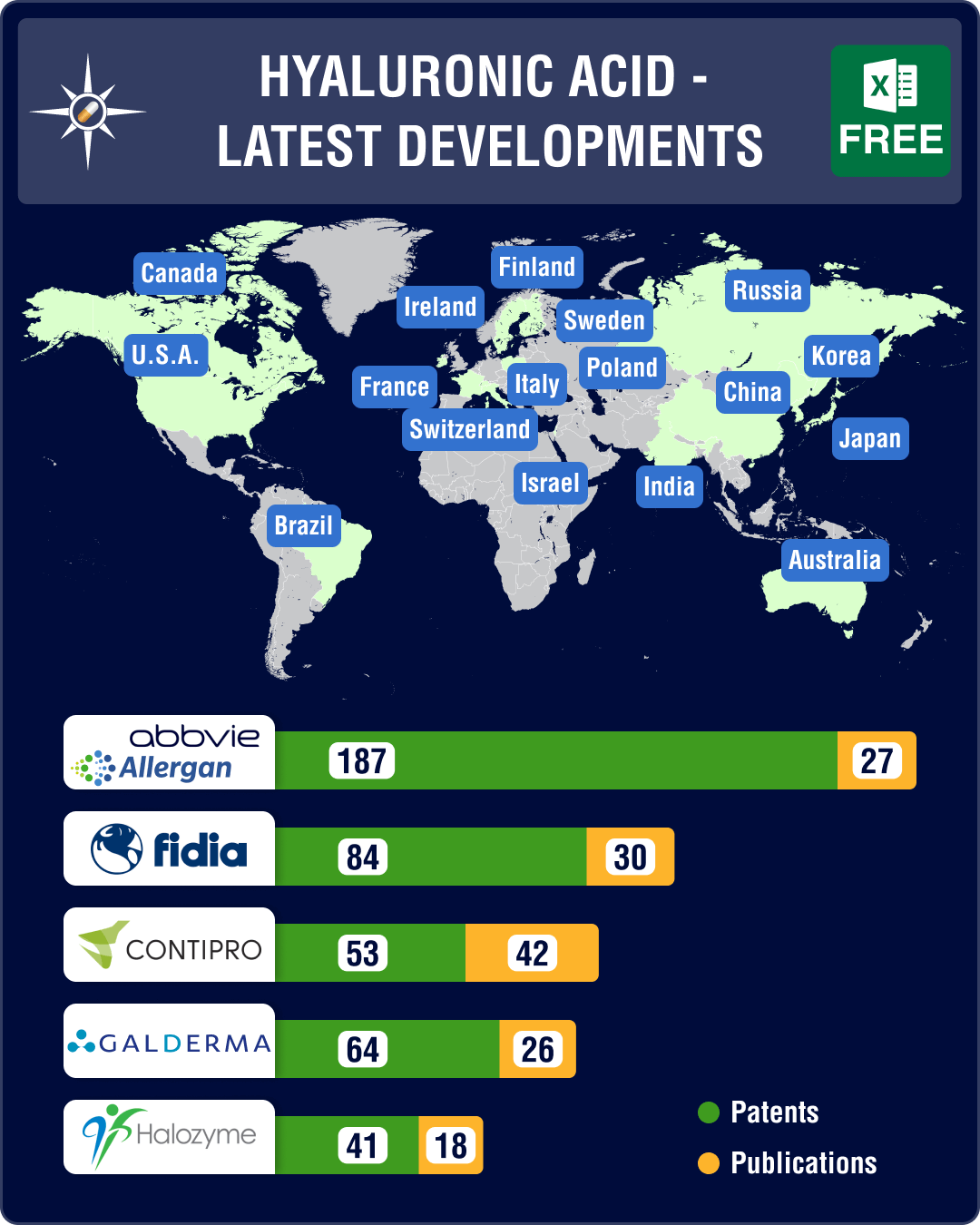
In view of the increased interest in sodium hyaluronate, we are leveraging PharmaCompass’ Technology Prospector, our proprietary technology, to provide an overview of organizations developing products linked to HA and sodium hyaluronate.
View Our Interactive Dashboard on Hyaluronic Acid (Free Excel Available)
HA — a naturally occurring biomacromolecule
Hyaluronic acid or hyaluronan has several health-boosting properties. Known for its hydrophilic and space filling characteristics, HA is directly or indirectly involved in every physiological function in the body.
Hyaluronan supplementation is needed due to reasons such as an injury, aging and disease, that lead to a decrease in HA concentration and molecular weight. Without proper levels of HA, mammals experience discomfort, reduced mobility, loss of tissue hydration, and other unwanted signs of aging.
HA is widely used in tissue-engineering due to its excellent biocompatibility, minor cross species variation, and ability to form flexible hydrogels. HA also helps with anti-inflammatory responses and immunosuppressive effects.
Sodium hyaluronate is commonly used in topical medications including serums and moisturizers for dermatological applications. Moreover, it is tipped to be a potential driving force in the upcoming beauty biotech revolution.
View Our Interactive Dashboard on Hyaluronic Acid (Free Excel Available)
Therapeutic applications of sodium hyaluronate
Orthopedics is a leading
therapeutic area for products containing sodium hyaluronate. Abiogen
leads with marketed products followed by Olea Pharma, ATGC, Bioiberica,
BMI Korea,
and Bone Therapeutics, which have drugs in various stages of development that
contain sodium hyaluronate.
Besides orthopedics, ophthalmology, dermatology, nutrition, musculoskeletal, bioprinting, hematology and otoprotection are some areas that use sodium hyaluronate in formulations. You can learn more about the global development landscape of these drugs by using PharmaCompass’ Technology Prospector tool.
Furthermore, Allergan has marketed products for ophthalmological and dermatological applications, Amsik Gmbh has marketed products for dermatological applications, and Bi Investment Llc / BioZyme Incorporated has marketed products for nutritional applications containing sodium hyaluronate.
View Our Interactive Dashboard on Hyaluronic Acid (Free Excel Available)
Big players in the business
There are several players who are working with products containing HA. Here, we have briefly profiled seven of them.
— Allergan, now a part of AbbVie, sells products containing sodium hyaluronate used for ophthalmologic applications as artificial tears to maintain hydration in eyes and for dermatological applications as fillers for cosmetic surgery.
It has various fillers
in its Juvederm portfolio — each injection contains a gel-like material made of water, HA, and lidocaine
(a local anesthetic).
Allergan is expanding its Refresh portfolio through the launch of its Refresh Relieva lubricant eye drop product line. All products in the portfolio contain carboxymethylcellulose, HA (an inactive ingredient), glycerin and HydroCell technology.
— Italian group Fidia Farmaceutici SpA is using HA in a big way. It’s a world leader in research, development, and manufacturing of HA-based products. Fidia signed a binding agreement with Sanofi for the acquisition of the French drugmaker’s anti-inflammatory drugs. It will acquire registrations, trademarks, and related commercial rights of seven products. Fidia has strengthened its position in ophthalmology and has also announced a new commercial agreement with Novartis for high-level products. Fidia’s wholly-owned subsidiary, Fidia Pharma USA Inc, has introduced Triluron in the US, an HA-based intra-articular viscosupplement indicated for the treatment of pain in osteoarthritis of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and to simple analgesics (e.g., acetaminophen).
— HTL,
a leading French supplier of HA and other biopolymer solutions, is investing in
a state-of-the-art production facility that will multiply its injectable grade sodium hyaluronate production capacity by 2.5 folds by 2021.
This new capacity represents a much-needed opening in a global market under
high pressure.
— Brinter Limited is a biopharmaceutical company which manufactures various bioprinters. Its modular Brinter platform built for 3D bioprinting of soft tissue constructs is capable of printing hydrogel bioinks for different use cases. Its publication describes the use of methacrylate hyaluronan as an ingredient in Bioink.
— Cellbricks Gmbh is a stereolithography-based bioprinter company which uses multiple HA-based photoinks in their printers. Stereolithography is a 3D printing process which uses a computer controlled moving laser beam that is pre-programmed using CAM/CAD software. Cellbricks produces structures containing embedded cells as well as cell-free biopolymer scaffolds using stereolithography.
— Decibel Therapeutics focuses on discovering and developing transformative treatments to restore and improve hearing and
balance. It is currently developing DB-020 as a novel formulation of sodium thiosulfate pentahydrate in 1 percent sodium hyaluronate for
intratympanic injection, enabling the delivery of high concentrations of
thiosulfate into the cochlea prior to cisplatin
administration. The clinical results, so far, support the design and execution of the ongoing proof-of-concept study — DB-020-002 — to assess otoprotection using DB-020 in cancer patients receiving cisplatin without negatively impacting cisplatin’s anti-tumor
efficacy.
—Shandong Topscience Biotech is one of the few manufacturers of sodium hyaluronate in China with a GMP certification. Its products include sodium hyaluronate, alpha-arbutin, ascorbyl glucoside, sulglycotide, etc., produced by microbial fermentation. It relies on raw materials from non-animal sources and has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
View Our Interactive Dashboard on Hyaluronic Acid (Free Excel Available)
Our view
Over the last few years,
the USFDA has granted approval to various drugs that use HA and sodium
hyaluronate, such as orthobiologic solutions, dermal fillers and ophthalmological products.
As per a report published by The Courier, the market for sodium hyaluronate is expected to register a compounded annual growth rate of around 4.2 percent over the next five years. This is due to the many applications of sodium hyaluronate in the industry today.
Given the increasingly sedentary lifestyle, rising screen times, the focus on beauty and the increasing reliance on bioprinting, sodium hyaluronate is one pharmaceutical ingredient that is bound to be in demand for years to come.
View Our Interactive Dashboard on Hyaluronic Acid (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Hyaluronic-Acid-Latest-Developments by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”