Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-Myers Squibb acquiring
New Jersey-based cancer drug company Celgene in a US$ 74 billion deal announced on
January 3, 2019. After factoring
in debt, the deal value ballooned to about US$ 95 billion, which according
to data compiled by Refinitiv, made it the largest healthcare deal on
record.
In the summer, AbbVie Inc,
which sells the world’s best-selling drug Humira, announced its acquisition of Allergan Plc, known for Botox and other cosmetic
treatments, for US$ 63 billion. While the companies are still awaiting
regulatory approval for their deal, with US$ 49 billion in combined 2019
revenues, the merged entity would rank amongst the biggest in the industry.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
The big five by pharmaceutical sales — Pfizer,
Roche, J&J, Novartis and Merck
Pfizer
continued
to lead companies by pharmaceutical sales by reporting annual 2019 revenues of
US$ 51.8 billion, a decrease of US$ 1.9 billion, or 4 percent, compared to
2018. The decline was primarily attributed to the loss of exclusivity of Lyrica in 2019,
which witnessed its sales drop from US$ 5 billion in 2018 to US$ 3.3 billion in
2019.
In 2018, Pfizer’s then incoming CEO Albert Bourla had mentioned that the company did not see the need for any large-scale M&A activity as Pfizer had “the best pipeline” in its history, which needed the company to focus on deploying its capital to keep its pipeline flowing and execute on its drug launches.
Bourla stayed true to his word and barring the acquisition of Array Biopharma for US$ 11.4 billion and a spin-off to merge Upjohn, Pfizer’s off-patent branded and generic established medicines business with
Mylan, there weren’t any other big ticket deals which were announced.
The
Upjohn-Mylan merged entity will be called Viatris and is expected to have 2020
revenues between US$ 19 and US$ 20 billion
and could outpace Teva to
become the largest generic company in the world, in term of revenues.
Novartis, which had
followed Pfizer with the second largest revenues in the pharmaceutical industry
in 2018, reported its first full year earnings after spinning off its Alcon eye
care devices business division that
had US$ 7.15 billion in 2018 sales.
In 2019,
Novartis slipped two spots in the ranking after reporting total sales of US$
47.4 billion and its CEO Vas Narasimhan continued his deal-making spree by buying New
Jersey-headquartered The Medicines Company (MedCo) for US$ 9.7
billion to acquire a late-stage cholesterol-lowering
therapy named inclisiran.
As Takeda Pharmaceutical Co was
busy in 2019 on working to reduce its debt burden incurred due to its US$ 62
billion purchase of Shire Plc, which was announced in 2018, Novartis also purchased
the eye-disease medicine, Xiidra, from the Japanese drugmaker for US$ 5.3 billion.
Novartis’ management also spent a considerable part of 2019 dealing with data-integrity concerns which emerged from its 2018 buyout of AveXis, the
gene-therapy maker Novartis had acquired for US$ 8.7 billion.
The deal gave Novartis rights to Zolgensma,
a novel treatment intended for children less than two years of age with the
most severe form of spinal muscular atrophy (SMA). Priced at US$ 2.1 million,
Zolgensma is currently the world’s most expensive drug.
However,
in a shocking announcement, a month after approving the drug, the US Food and
Drug Administration (FDA) issued a press release on
data accuracy issues as the agency was informed by AveXis that
its personnel had manipulated data which
the FDA used to evaluate product comparability and nonclinical (animal)
pharmacology as part of the biologics license application (BLA), which was
submitted and reviewed by the FDA.
With US$
50.0 billion (CHF 48.5 billion) in annual pharmaceutical sales, Swiss drugmaker
Roche came in at number two position in 2019
as its sales grew 11 percent driven by
its multiple sclerosis medicine Ocrevus, haemophilia drug Hemlibra and cancer medicines Tecentriq and Perjeta.
Roche’s newly introduced medicines generated US$ 5.53 billion (CHF 5.4 billion) in growth, helping offset the impact of the competition from biosimilars for its three best-selling drugs MabThera/Rituxan, Herceptin and Avastin.
In late 2019, after months of increased
antitrust scrutiny, Roche completed
its US$ 5.1 billion acquisition of Spark Therapeutics to strengthen its presence in
gene therapy.
Last year, J&J reported almost flat worldwide sales of US$ 82.1 billion. J&J’s pharmaceutical division generated US$ 42.20 billion and its medical devices and consumer health divisions brought in US$ 25.96 billion and US$ 13.89 billion respectively.
Since J&J’s consumer health division sells analgesics, digestive health along with beauty and oral care products, the US$ 5.43 billion in consumer health sales from over-the-counter drugs and women’s health products was only used in our assessment of J&J’s total pharmaceutical revenues. With combined pharmaceutical sales of US$ 47.63 billion, J&J made it to number three on our list.
While the sales of products like Stelara, Darzalex, Imbruvica, Invega Sustenna drove J&J’s pharmaceutical business to grow by 4 percent over 2018, the firm had to contend with generic competition against key revenue contributors Remicade and Zytiga.
US-headquartered Merck, which is known as
MSD (short for Merck Sharp & Dohme) outside the United States and
Canada, is set to significantly move up the rankings next year fueled by its
cancer drug Keytruda, which witnessed a 55
percent increase in sales to US$ 11.1 billion.
Merck reported total revenues of US$ 41.75 billion and also
announced it will spin off its women’s health drugs,
biosimilar drugs and older products to create a new pharmaceutical
company with US$ 6.5 billion in annual revenues.
The firm had anticipated 2020 sales between US$ 48.8 billion and US$ 50.3 billion however this week it announced that the coronavirus pandemic will reduce 2020 sales by more than $2 billion.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Humira holds on to remain world’s best-selling drug
AbbVie’s acquisition of Allergan comes as the firm faces the expiration of patent protection for Humira, which brought in a staggering US$ 19.2 billion in sales last year for
the company. AbbVie has failed to successfully acquire or develop a major new
product to replace the sales generated by its flagship drug.
In 2019, Humira’s US revenues increased 8.6 percent to US$ 14.86 billion while internationally, due
to biosimilar competition, the sales dropped 31.1 percent to US$ 4.30 billion.
Bristol Myers Squibb’s Eliquis, which is also marketed by Pfizer, maintained its number two position
and posted total sales of US$ 12.1 billion, a 23 percent increase over 2018.
While Bristol Myers Squibb’s immunotherapy treatment Opdivo, sold in partnership with Ono in Japan, saw sales increase from US$ 7.57 billion to US$ 8.0 billion, the growth paled in comparison to the US$ 3.9
billion revenue increase of Opdivo’s key immunotherapy competitor Merck’s Keytruda.
Keytruda took the number three spot in drug sales that
previously belonged to Celgene’s Revlimid, which witnessed a sales decline from US$ 9.69 billion to US$ 9.4 billion.
Cancer treatment Imbruvica, which is marketed
by J&J and AbbVie, witnessed a 30 percent increase in sales. With US$ 8.1
billion in 2019 revenues, it took the number five position.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Vaccines – Covid-19 turns competitors into partners
This year has been dominated by the single biggest health emergency in years — the novel coronavirus (Covid-19) pandemic. As drugs continue to fail to meet expectations, vaccine development has received a lot of attention.
GSK reported the highest vaccine sales of all drugmakers with
total sales of US$ 8.4 billion (GBP 7.16 billion), a significant portion of its
total sales of US$ 41.8 billion (GBP 33.754 billion).
US-based Merck’s vaccine division also reported a significant increase in sales to US$ 8.0 billion and in 2019 received FDA and EU approval to market its Ebola vaccine Ervebo.
This is the first FDA-authorized vaccine against the deadly virus which causes
hemorrhagic fever and spreads from person to person through direct contact with
body fluids.
Pfizer and Sanofi also reported an increase in their vaccine sales to US$ 6.4
billion and US$ 6.2 billion respectively and the Covid-19 pandemic has recently
pushed drugmakers to move faster than ever before and has also converted
competitors into partners.
In a rare move, drug behemoths — Sanofi and GlaxoSmithKline (GSK) —joined hands to develop a vaccine for the novel coronavirus.
The two companies plan to start human trials
in the second half of this year, and if things go right, they will file
for potential approvals by the second half of 2021.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Our view
Covid-19 has brought the world economy to a grinding halt and shifted the global attention to the pharmaceutical industry’s capability to deliver solutions to address this pandemic.
Our compilation shows that vaccines and drugs
for infectious diseases currently form a tiny fraction of the total sales of
pharmaceutical companies and few drugs against infectious diseases rank high on
the sales list.
This could well explain the limited range of
options currently available to fight Covid-19. With the pandemic currently infecting
over 3 million people spread across more than 200 countries, we can safely
conclude that the scenario in 2020 will change substantially. And so should our
compilation of top drugs for the year.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Impressions: 54752
Now that the World
Health Organization (WHO) has declared the new coronavirus (2019-nCoV) as a public health emergency of
international concern, some of the biopharma industry’s
biggest names are taking important steps to combat the disease.
The death toll from the coronavirus outbreak
is rising each day. As many as 563 people have died in China, and the
virus has now spread to nearly 30 countries across the world, including Japan,
Thailand, Singapore, Hong Kong, South Korea, Australia, Malaysia, Germany,
India and the US.
In fact, infectious disease specialists and
scientists say the new coronavirus may be more contagious than what the current data suggests. The virus has spread from about 300 people on January 21
to over 28,000 people across the world. The numbers may be even higher as there
is limited testing capacity.
“The rapid acceleration of cases is of concern,” Dr. Mike Ryan, executive director of the WHO’s emergencies program said last week.
Health authorities all over the world are trying
to contain the outbreak. In the US, nearly 200 Americans were placed under
quarantine at a US air base in California after being evacuated from Wuhan,
China. Under the new restrictions, US citizens who
have traveled to China within the last 14 days will be directed to one of the
11 airports designated for screening. The FDA is halting travel of all its
employees to China and recalling its inspectors from the country.
Shortage of face masks, body suits
The fear of the fast spreading coronavirus has
led to an acute shortage of face masks in
Asia, the US and the UK. Manufacturers are increasing production to meet
surging demand.
In fact, China is the source
of most of the world’s masks and respirators. However, today it is using more masks than
it ever has. As a result, fewer masks and respirators will be available to the countries
that have been importing them from China.
In fact, former FDA commissioner Scott Gottlieb tweeted that China is witnessing a shortage of protective body suits. “Since much of supply chain for masks, gowns, gloves is through China; we should prepare for potential of stretched supply chains and increased demand here in US in event of outbreaks or epidemic spread in America,” Gottlieb tweeted quoting a Bernstein analysis
Meanwhile, India has banned the export of personal
protection equipment such as masks and clothing amid the global coronavirus
outbreak.
In Hong Kong, which has been struggling with a
shortage of face masks, toilet rolls became unavailable in some
supermarkets. Social media posts showed empty shelves and shoppers
lining up to buy rolls.
Success of flu, HIV drugs on coronavirus: Though there is no formal cure discovered to treat the virus, the
treatment protocol established in China and confirmed by physicians in Thailand
shows that a combination of Lopanvir and Ritonavir,
old generation drugs used to treat HIV, along with flu medication Oseltamivir,
has worked on patients.
Thai doctors have seen success of such a treatment. “This is not
the cure, but the patient’s condition has vastly improved. From testing positive for 10 days under our care, after applying this combination of medicine the test result became negative within 48 hours,” Dr. Kriangska Atipornwanich, a lung specialist at Rajavithi Hospital in Bangkok said.
Thailand has recorded 19 cases of coronavirus.
Of the Thai patients, eight have recovered and gone home while 11 are still
under treatment in hospitals.
Meanwhile, Indian drug companies say they are ready with supply of
anti-retrovirals (ARVs) that seem to work in treating the novel coronavirus.
Rise in screening tests: Roche has
developed a test that can tell whether or not someone has the 2019-nCoV
infection within a couple of hours. Roche’s coronavirus test hasn’t been
approved for marketing as yet.
Co-Diagnostics Inc has also said that the
initial verification of its screening test designed to identify the presence of
the coronavirus was successful.
Healthcare company Novacyt has also launched a
new molecular test for the Wuhan coronavirus.
The company said its Primerdesign coronavirus
test could detect the 2019 strain of the virus, adding it believed this would
differentiate it from other current tests which it said were less specific.
New coronavirus projects
There are multiple drugmakers and diagnostics
companies making noise about their coronavirus projects. Here are some
noteworthy projects:
Gilead’s remdesivir: Gilead’s experimental anti-viral treatment — remdesivir — has shown some positive results as
a patient in the US with worsening symptoms of a confirmed 2019-nCoV infection
has been treated with the drug. Remdesivir
appeared effective as a throat swab was tested negative for the virus just a
few days after receiving the infusion.
Incidentally, Gilead is developing remdesivir to combat the Ebola virus, not 2019-nCoV. Success from a single patient is great news, but the company needs more evidence to prove its case.
Gilead is also expediting laboratory testing of
remdesivir against samples of the new coronavirus. Last week, Anthony Fauci, director of the US
National Institute of Allergy and Infectious Diseases, said his agency was
working with Gilead to test remdesivir.
Gilead is also working with Chinese authorities
to begin a controlled, randomized study. However, such a study is bound to take
months.
Meanwhile, China has kick-started a clinical
trial to speedily test remdesivir for 2019-nCoV.
In fact, the virology institute in Wuhan has applied for a patent on this experimental
Gilead drug. Scientists have found remdesivir and chloroquine, an 80-year-old
malaria drug, to be highly effective in laboratory settings to thwart the novel
coronavirus. However, the two drugs’ efficacies
on humans required further clinical tests, the institute said.
Remdesivir
will also be tested by a medical team from Beijing-based
China-Japan Friendship Hospital for efficacy in treating the deadly new strain
of coronavirus.
Gilead said the drug was shown to be active in
animals with Severe Acute Respiratory Syndrome (SARS) and Middle East
Respiratory Syndrome (MERS), which are closely related to the current virus.
Moderna, J&J, GSK work on vaccines: Moderna, a
Massachusetts-headquartered biotechnology firm, is developing a vaccine to
prevent the coronavirus from spreading. Though there isn’t any accomplishment as yet, the Coalition for Epidemic Preparedness
Innovations (CEPI) has agreed to fund the manufacturing of a 2019-nCoV vaccine
that uses the company’s
proprietary messenger RNA (mRNA) platform.
Last week, Johnson & Johnson also said it would begin work on developing a vaccine for the
virus.
GlaxoSmithKline and CEPI also said they would work to accelerate the creation of
a vaccine. The goal is then to provide many vaccine doses rapidly, a statement
said.
The project will rely on GSK’s adjuvant
system, designed to enhance the body’s immune response and create a stronger and longer lasting
protection against infection. However, developing a preventive and confirming
that it is safe and useful in humans could take 12 to 18 months
Regulators, WHO prepare for new cures
The European Medicines Agency (EMA), US Food and
Drug Administration (FDA) and the WHO have taken various precautions and are preparing
themselves for new medicines, vaccines and in-vitro diagnostics (IVDs).
FDA has issued an Emergency Use Authorization (EUA) to authorize the emergency use of Centers for Disease Control and Prevention's (CDC) 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel for the “presumptive qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens (such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) collected from individuals who meet CDC criteria for 2019-nCoV testing.”
To date, this test has been limited to use at CDC laboratories, but FDA said today’s authorization allows the use of the test at any CDC-qualified lab nationwide.
Similarly, EMA said it is ready to support drug developers with all available regulatory tools (i.e., scientific advice, the PRIME scheme, the accelerated assessment and conditional marketing authorization) to advance and expedite the development of ways to fight and prevent the spread of this virus.
WHO says ‘no effective treatment’ yet
The WHO has played down reports that talk of effective drugs that could treat the new coronavirus. Television reports in China have pointed to an effective drug for the virus. And Britain’s Sky News said researchers had made a “significant breakthrough” in developing a vaccine.
WHO spokesman Tarik Jasarevic said: “There are no known effective therapeutics against this 2019-nCoV (virus) and the WHO recommends enrollment into a randomized controlled trial to test efficacy and safety.”
“Even at the accelerated pace enabled by new technologies, the earliest that scientists hope to be able to start initial human trials of a new coronavirus vaccine is by June this year,” a Reuters news report said.
Our view
According to the WHO website, there have been multiple disease outbreaks over the last 20 years, including diseases like dengue, avian flu, zika virus, SARS, MERS and many others. Till date, most of these diseases don’t have a cure or a vaccine. These diseases are being treated by various antiviral drugs, and the treatments are largely symptomatic. “The process of developing and testing drugs or vaccines against a new pathogen normally takes many years and is often fraught with pitfalls and failures,” a Reuters report said.Most of the viruses are mutating at a fast pace, creating newer strains each year. Scientists are concerned that the 2019-nCoV has mutated to adapt to its new human hosts far more quickly than SARS. Data on the virus is changing by the day, and some infectious disease specialists say it will take weeks before they can see just how contagious it is. Scientists believe the virus is more contagious than what the current data suggests.Clearly then, this is a race between a fast-spreading and fast-mutating virus and the skills of the biopharmaceutical industry to contain its spread. This time though, the pharmaceutical industry is showing a lot of urgency in finding a vaccine as well as a cure.
Impressions: 2136
The new coronavirus—named 2019-nCoV—has raised concerns across the world, and the reported cases and deaths due to mysterious pneumonia is doubling every couple of days. So far, 170 people in
China have died due to this new virus, and 7,711 confirmed cases of
the virus have been reported in the country. In fact, according to a
BBC report, the virus has now spread to every region in mainland China.
Moreover, there have been more than 80 cases of the virus reported outside mainland China. Deaths and cases are likely to rise until the outbreak is contained.
According to a CNN
report, the number of confirmed cases of the Wuhan
coronavirus has overtaken the 2003 SARS outbreak inside of mainland
China. Patients
of the mysterious disease
have now been reported from many Asian countries, as well as the
Middle East, Europe, Australia and the US.
During the 2003
SARS outbreak, there were 5,327 confirmed cases of the disease in
mainland China, with 349 deaths. In comparison, the Wuhan virus has
reported nearly 7,800 cases worldwide. Experts
say the Wuhan virus figures
could be vastly under-reported, making the novel coronavirus far more
contagious, though less deadly than SARS.
China on
high-alert
The outbreak has put health authorities on high alert in China and
around the world.
The
alarm is an outcome of lack of adequate information on the newly
identified coronavirus. Questions still linger on how dangerous is
this virus and how easily it can spread from one person to another.
The incubation period for the virus can range from one to 14 days. During this period, the patient can acquire pneumonia which has proven to be fatal in several recent cases in China.
Most of the deaths have been in the Hubei province of China, whose capital is Wuhan, a city whose population is larger than that of New York, London and Hong Kong. Chinese authorities have restricted some travel to try and stop the illness from spreading during the Lunar New Year, which started on January 25. The holiday break has been extended for three more days (until February 2), and China’s financial markets will also remain closed until then. The Shanghai government has said that companies in the city are not allowed to resume operations before February 9.
Countries across the world are also taking all sorts of precautions. In the US, the Centers for Disease Control and Prevention (CDC) said it is closely monitoring the
outbreak. In Singapore, a multi-ministry
task force was set up to
fight the disease on all fronts.
According to Scott
Gottlieb, former commissioner of the US Food and Drug Administration,
the epidemic in China is likely to be much broader than what
the official statistics currently suggest. And a lot of mild cases
have probably gone unrecognized. Moreover, he feels that a global
spread of the virus is inevitable.
While
the World Health Organization stopped short of calling the outbreak a
global health emergency, discussions are currently underway which may
change that status.
Researchers look
for cure
Health authorities
are exploring
repurposing
untested antivirals in a desperate bid to
contain the outbreak.
Drugmakers in the US are shipping antiviral drugs to Chinese health
authorities to assess whether these medicines could help contain the
spread of this disease.
A Chinese government commission has recommended that doctors administer Kaletra — AbbVie’s HIV drug — to patients who have been tested positive for the new illness. Kaletra is a combination of lopinavir
and ritonavir.
The other treatment is nebulized alpha-interferon.
AbbVie and Johnson & Johnson have begun
shipping drugs approved to treat HIV. AbbVie
is
donating
more than US$ 1
million worth of Kaletra to
help combat the disease in China.
There is uncertainty over whether drugs approved to treat other infectious diseases would help patients infected with the new coronavirus.
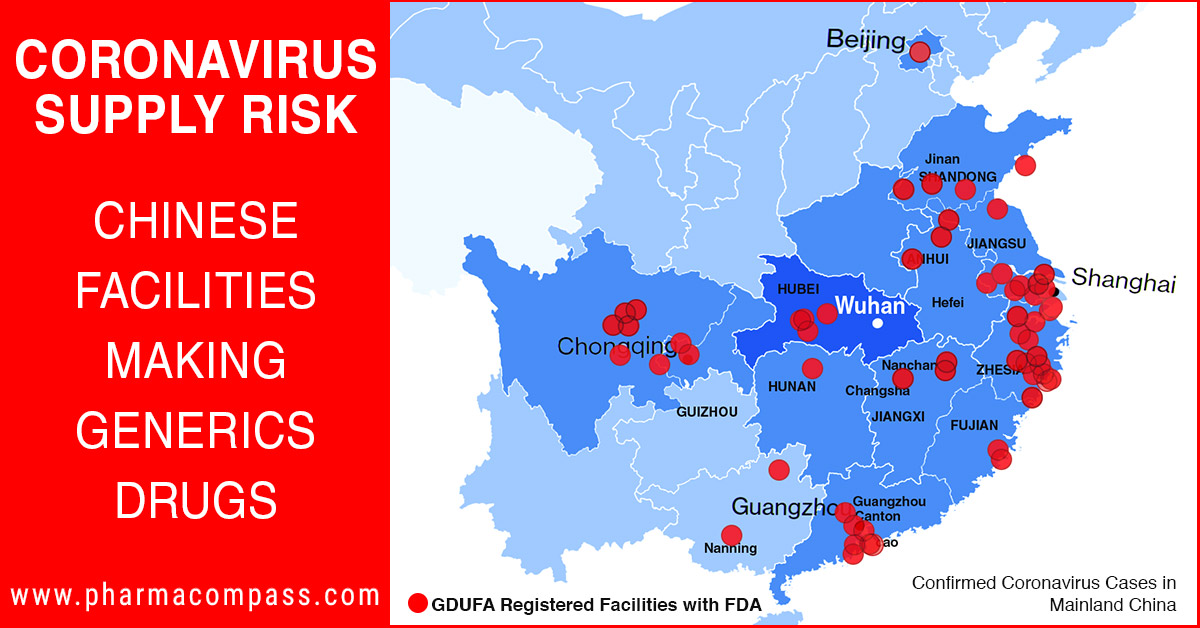
Disrupting API
supplies
As the virus proliferates and takes more lives, questions are likely to be raised about the adequate production and supplies of APIs, as well as the extent to which shipments can be made if transit hubs are out of commission.
There are a limited
number of generic drug facilities registered with the FDA
in the Hubei province. However, as the containment initiatives spread
across China, especially near the regions around Shanghai and
Guangzhou, the disruption possibilities are likely to increase
substantially.
In the US, approximately 80 percent of APIs
used to produce finished products come from China and other non-TAA
compliant countries. The
production cost of APIs in China is
estimated to be about 20 to 30 percent lower than that in
India. According to
reports, India
imports around 80 per cent of APIs from China on volume basis. So the world’s reliance on China for APIs is very high.
“The time to worry is now,” said Steven Lynn, a former director of the FDA Office of Pharmaceutical Quality. As of now, FDA has said it has not received any shortage notifications from manufacturers that make products for the US market due to the current coronavirus outbreak.
Our view
The spread of this new virus has bearings on the global supply chain for pharmaceuticals. Over the past decade, China has become an even bigger player in the market for antibiotics and the key building blocks of APIs.
The global
pharmaceutical supply chain is extremely interlinked and a lot of
pharmaceutical manufacturing is dependent on chemical and excipient
producers which are not listed on the generic facilities list.
As there is
restricted movement within parts of China, companies need to assess
their supply chains in detail to determine the likelihood of
disruption. They also need to speak to producers outside China to
build the necessary contingencies in case this problem is not
resolved quickly.
Impressions: 4479
In Phispers this week, there is news on how alternatives to Mylan’s EpiPen have eaten into its US sales. Warning letters posted by the FDA revealed that it considers seven of Wockhardt’s sites out of compliance and there is more trouble in the works for the former Ranbaxy promoters. And, Novartis faces punitive action in South Korea. Read on.
Mylan’s EpiPen sees drastic drop in market share in Jan-Feb 2017
Last year, Mylan was in
the news for hiking the price of EpiPen by
500 percent. At that time, there were no generic avatars of the
life-saving anti-allergy medicine available in the US market. But now, the
scenario has changed. Data from athenahealth network shows that prescriptions for alternatives of Mylan’s EpiPen have “quadrupled since the beginning of 2017”.
More than 60,000 prescriptions
for epinephrine auto-injectors, written for 50,000 patients by more than 1,400
healthcare providers in the US, were evaluated by researchers at athenahealth
between January 2016 and February 2017. The share of alternatives to
EpiPen have significantly increased in the last two months, as opposed to the
end of 2016.
“At the end of 2016, EpiPen alternatives made up only 5.3 percent of prescriptions for epinephrine auto-injectors. By the last full week of February, that figure had grown to 28.9 percent,” athenahealth said.
The key reason for this sudden and significant shift is the lower price tags of the EpiPen alternatives. Most of these alternatives are priced at a fraction of the cost of Mylan’s EpiPen. Moreover, insurers could also be affecting the prescription patterns. In January this year, health insurance company Cigna began to cover only generic options to EpiPen.
Seven of your facilities are out of compliance, FDA tells
Wockhardt
This week, US FDA posted
the warning letter issued to Wockhardt's US-based Morton Grove facility. The concerns raised by the investigators ranged from Wockhardt's “investigations into out-of-specification (OOS) test results” that were found to be neither thorough, nor timely, nor based on scientific rationales” to the “original results” that were “invalidated without scientific justification under the protocol and only re-test results were reported as part of batch release decisions.”
Of serious concern is the fact that in the warning letter the FDA has said: "At this time, seven Wockhardt facilities (including Morton Grove) are considered out of compliance with cGMP. These repeated failures at multiple sites demonstrate your company’s inadequate oversight and control over the manufacture of drugs. In your responses to the various actions listed above, including during multiple meetings with FDA, you have repeatedly discussed and promised corporate-wide corrective actions. Yet, when FDA inspects or returns to other Wockhardt facilities, similar violations are shown to persist.”
Second FDA warning letter to Megafine over "calibration records in trash”
Megafine
Pharma received its second
FDA warning letter in less than a year for its facility which manufactures active pharmaceutical ingredient (API) intermediates. The Megafine plant in Vapi (Gujarat) was inspected last September and once again data
integrity was the primary cause of concern raised by the FDA.
The facility was “issuing analysts with blank controlled document forms that had already been approved and signed”. Moreover, FDA investigators found torn, partially complete Quality Assurance-signed calibration records in the trash and saw QA staff shredding documents without reason.
In another observation, "analysts reprocessed data up to 12 times, and only included the final result in the report for review by Quality Assurance. Your Deputy Manager, Quality Control stated that it is common practice to ‘play with parameters’ to get the proper integration."
FDA investigators also noticed that the interior surfaces of the
drug manufacturing equipment were not as clean as required.
Megafine's API plant in Lakhmapur, Nashik, was placed on FDA's Import
Alert list in 2015.
Ghosts of Ranbaxy continue to haunt Singh brothers
Former promoters of Ranbaxy — Malvinder and Shivinder Singh — were directed by the Delhi High
Court to seek its permission before selling any of their unencumbered assets. This puts in jeopardy reported plans of the brothers to sell their stakes in companies like Fortis Healthcare and Religare, although the court hasn’t passed an outright injunction against such an exercise.
The Singh brothers have also
been directed to furnish details of all unencumbered assets in order to ascertain
that they have enough funds to cover an arbitration payment of more than US$
3.74 billion (Rs 250 billion) granted to Daiichi Sankyo by a Singapore tribunal
last year.
The brothers have been engaged
in a legal battle with Daiichi Sankyo, which bought out Ranbaxy in 2008 for US$
4.1billion. Daiichi had accused the Singh
brothers of misrepresenting the problems facing Ranbaxy when it acquired the
firm. In 2014, Daiichi sold Ranbaxy to Sun Pharma.
Investors demand cut in new GSK CEO’s prospective pay package
A number of investors in Britain’s largest drug company — GlaxoSmithKline (GSK) — are reportedly putting pressure on its board to reduce a proposed multi-million pound pay deal for its
new CEO, Emma Walmsley. They want Walmsley’s package to be sufficiently lower than the one awarded to her predecessor — Sir Andrew Witty — who is slated to retire later this month. His base salary is US$ 1.34 million (£1.1million) a year, while Walmsley’s proposed base salary is US$ 1.22 million (£1 million) a year, said news reports.
Some shareholders don’t think her proposed pay package fits Walmsley’s work experience. She is set to become Big Pharma’s first female CEO. But her resume is heavily tilted towards consumer products. Till recently, she was heading GSK’s consumer health joint venture. And prior to 2010 (a year when she joined GSK), she spent 17 years at cosmetics company — L’Oreal.
GSK is in “active consultations with shareholders” and is “acutely aware of the need for a balanced and responsible approach to remuneration,” a spokesperson said.
Children with AIDS write to India’s PM about Cipla’s Lopinavir
When the Indian government
failed to clear its dues, Cipla — the only manufacturer of Lopinavir syrup (a child-friendly HIV drug) — stopped
production of the drug. As a
result, children living with HIV (CLHIV) have written to Prime Minister
Narendra Modi for help.
The letter dated March 4 is
signed by 637 children ranging from ages three to 19. “The pharmaceutical company Cipla has in various forums cited delay in payments by the national program for the HIV medicines by several years and even non-payment of its dues in many cases. Profits on child doses of HIV medicines are small and delayed payments are having a chilling effect on the ability of the National AIDS Control Organization (NACO) to convince the company to participate in the bids it invited annually,” the letter said.
Cipla is the dominant player in
the Indian market across the HIV segment. Even though the Indian government has
failed to pay Cipla for consignments it had sent back in 2014, the company has
not stopped participating in government tenders.
The Health Ministry, on the other hand, says it has instructed State AIDS Control Societies (SACS) to purchase the drug from local markets. “An emergency tender has been placed but we have instructed SACS and state governments to purchase from local markets,” Arun Panda, Additional Secretary, Health Ministry, said.
Novartis faces drug ban in
South Korea over bribe issue
Novartis is
due to face additional
punitive measures from the health
ministry of South Korea later this month for allegedly providing illegal bribes
to local doctors in exchange for prescribing its drugs.
Last week, the Korean Ministry
of Health and Welfare said it is reviewing its own administrative actions
against Novartis Korea, which could take the form of a fine or a lifting of
insurance benefits on drugs sold by the company in South Korea. Novartis is
headquartered in Basel, Switzerland.
Last week, the Korean Ministry
of Food and Drug Safety had slapped Novartis Korea with a fine of US$ 174,937 (200 million won) and a three-month sales ban on 12 drugs, including variations of the company’s flagship dementia drug Exelon.
According to
the ministry, this 200 million-won fine is equivalent to a three-month ban on 30
drugs, including Novartis’ top-selling diabetes drug — Galvus.
Korea’s punitive measures against Novartis Korea have come a year after Seoul prosecutors began a probe into a massive bribery scam that involved the company’s executives and employees.
In August last year, six former and current Novartis employees in South
Korea were indicted over illegal practices to boost sales of the company's
drugs.
Samsung Bioepis wins high-stakes case against AbbVie’s Humira
There is more news from South
Korea. The Seoul-headquartered Samsung Bioepis, a biopharmaceutical company, won a high-stakes court case last week against US drug firm AbbVie for a biosimilar of the world's best-selling drug — Humira.
Samsung
is keen to sell a version of this
arthritis treatment in Europe sometime early next year. The biosimilar version
of Humira is pending approval by the European Medicines Agency (EMA).
This ruling of the England and Wales High Court Chancery Division of the Patents Court, while determining that the two patents covering AbbVie’s Humira are invalid, also said that AbbVie's indications pertaining to rheumatoid arthritis, psoriasis and psoriatic arthritis were unpatentable.
The other
claimant in this case is Tokyo-based Fujifilm Kyowa Biologics. In 2015, Humira
displaced Pfizer’s anti-cholesterol drug Lipitor by crossing US$ 14 billion in
sales.
Impressions: 3064