By PharmaCompass
2023-07-06
Impressions: 2829
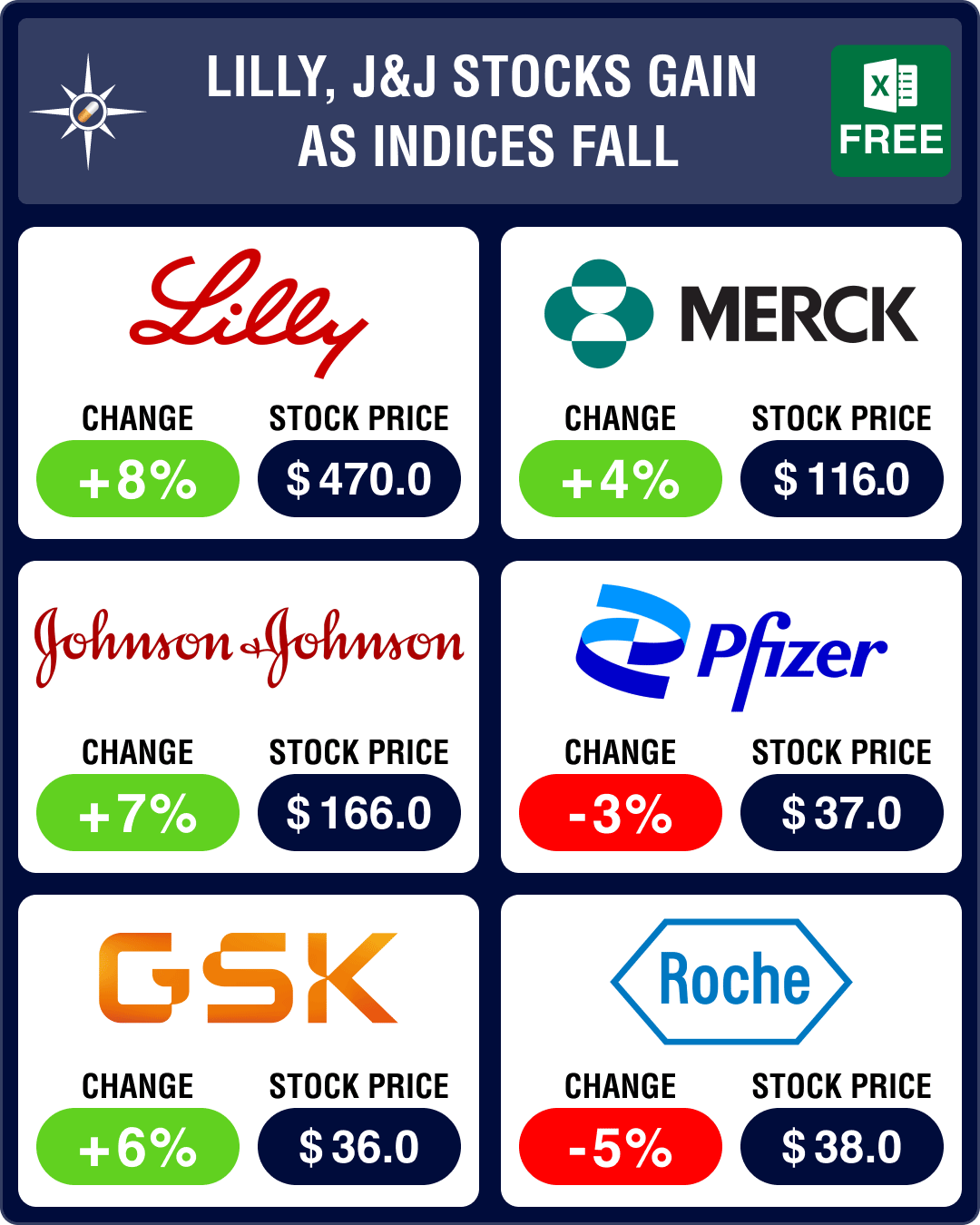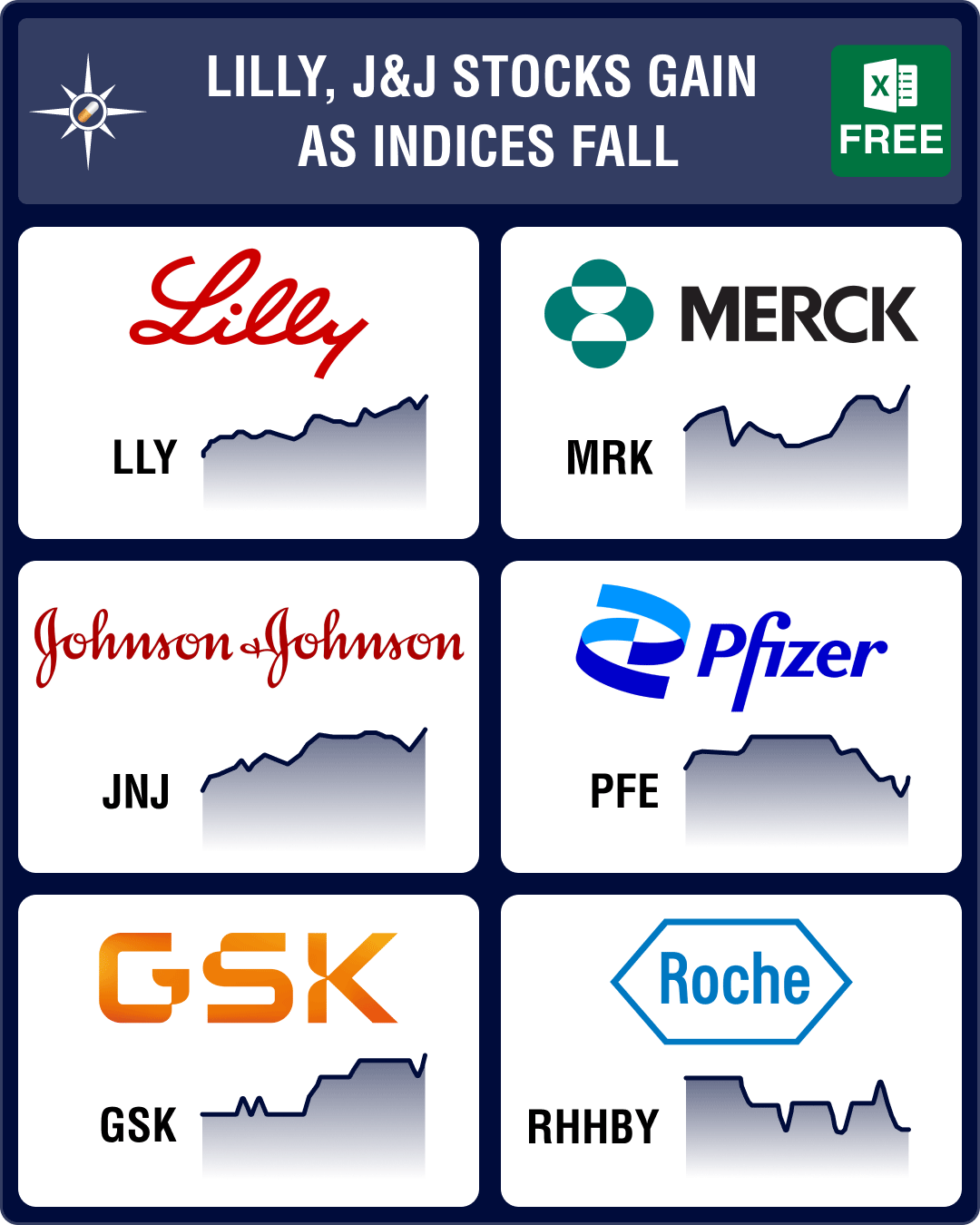
June was a lackluster month for pharma stocks. The Nasdaq Biotechnology Index (NBI) was down 0.7 percent at US$ 4,065.95, against a 4 percent drop in May (at US$ 4054.54). SPDR S&P Biotech ETF index (XBI) was down 1.8 percent at US$ 83.95, against a 2 percent rise in May, and the S&P Biotechnology Select Industry Index (SPSIBI) was down 2.9 percent at US$ 6,409.05, against a 1 percent rise in May. Even though the indices slipped into red, several mega cap drugmakers like Eli Lilly, Johnson & Johnson (J&J) and Sanofi saw their stocks rise.
There was a pick-up in dealmaking. Among the bigger deals were Novartis’ (up 4 percent) acquisition of American biotech Chinook Therapeutics (up 56 percent) for up to US$ 3.5 billion, Eli Lilly’s acquisition of Dice Therapeutics (up 47 percent) for around US$ 2.4 billion and Bausch + Lomb’s (up 22 percent) buyout of several eye-care products from Novartis for up to US$ 2.5 billion.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
Weight loss med boosts Lilly’s stock; concerns over obesity drug drag Pfizer down
Eli Lilly’s (up 8 percent) stock reached an all-time high after the company set a new bar among weight loss drugs with retatrutide (a GIP, GLP-1 and glucagon receptor triple agonist). In a phase 3 trial, the drug led to an average 24.2 percent reduction in weight over 48 weeks in patients without diabetes.
The market has high expectations from this class of obesity drugs. That’s why it was unkind to Pfizer (-3 percent) when it announced it had discontinued the development of its experimental obesity and diabetes drug — lotiglipron (GLP-1 receptor agonist) — due to safety concerns. Pfizer’s market cap took a nearly US$ 12 billion hit on this news. The drug behemoth is shifting focus to its other obesity drug — danuglipron.
In approvals, the US Food and Drug Administration (FDA) approved Lilly and Boehringer Ingelheim’s Jardiance for the treatment of type 2 diabetes in children 10 years and older. This is the first and only SGLT2 inhibitor approved for this patient population.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
J&J gains on new Carvykti data; Merck’s Lynparza scores win in prostate cancer trial
J&J and Legend Biotech filed for expanded use of Carvykti after presenting remarkable data from a phase 3 trial in relapsed and lenalidomide-refractory multiple myeloma. The drug was previously administered after four or more lines of therapy. Now, the companies hope to get approval for those who have received at least one prior line of therapy. With such an approval, Carvytki could get ahead of BMS’ multiple myeloma CAR-T therapy, Abecma, which was approved for fourth-line use in 2021.
J&J also gained due to results from a phase 2 combination study of talquetamab and Tecvayli in relapsed or refractory multiple myeloma. Overall, J&J’s stock was up 7 percent in June, while Legend Biotech’s stock went up 11 percent.
Another gainer was Merck (stock up 4 percent). Its Lynparza (olaparib) plus abiraterone combination has been approved by the FDA for the treatment of BRCA-mutated metastatic castration-resistant prostate cancer. The approval is based on data from a phase 3 trial, where the drug combo reduced the risk of disease progression or death by 76 percent compared to abiraterone alone in patients. With this approval, Lynparza has become the first poly ADP ribose polymerase (PARP) inhibitor to be approved in combination with a novel hormonal agent.
Among mega-cap losers was Roche (-5 percent) whose PD-L1 inhibitor Tecentriq and Exelixis’ Cabometyx not only failed to offer any additional benefit in previously treated kidney cancer but also added toxicity.
Among small cap gainers, Black Diamond Therapeutics saw its stock rise 150 percent as it announced phase 1 trial data on BDTX-1535 that showed positive results in non-small cell lung cancer (NSCLC) patients.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
GSK gains from Zantac settlement; FDA panel backs Astra-Sanofi’s RSV drug
GSK (up 6 percent) reached its first legal settlement in litigation against its heartburn medication Zantac (ranitidine). This may set a precedent for thousands of other ranitidine cases set to go to trial next year.
In another Zantac lawsuit, an International Chamber of Commerce tribunal rejected Boehringer Ingelheim’s attempt to gain indemnification from Sanofi for future liabilities arising from lawsuits in the US from users of the heartburn drug. Sanofi (up 6 percent) had acquired Zantac through an asset swap with Boehringer in 2016.
There were updates on respiratory syncytial virus (RSV) vaccines. The US Centers for Disease Control and Prevention (CDC) recommended that Pfizer’s Abrysvo and GSK’s Arexvy vaccines be used to prevent severe RSV infections in older adults.
Similarly, an FDA panel voted unanimously that AstraZeneca (-1 percent) and Sanofi’s nirsevimab has a favorable benefit-risk profile for the prevention of RSV lower respiratory tract disease in newborns and infants. If approved, nirsevimab would be the first preventive option for newborns and infants. The drug is likely to get an FDA nod in the third quarter of this year.
FDA has accepted the biologics license applications (BLAs) submitted by Vertex Pharmaceuticals (up 9 percent) and CRISPR Therapeutics for the investigational treatment exagamglogene autotemcel (exa-cel) to treat severe sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). FDA has granted a priority review for SCD and a standard review for TDT.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
Doubts over Leqembi hits Biogen stock; Regeneron loses due to rejection of higher-dose Eylea variant
Alzheimer’s disease experts in Europe raised doubts over the clinical benefits of Biogen and Eisai’s Leqembi. They said the benefits of Leqembi may not outweigh its potential side effects and the cost to the health system. This led to a 7 percent dip in Biogen’s stock.
Last month, an FDA advisory panel unanimously agreed that a late-stage trial of Leqembi had verified the benefit of the treatment for those at an early stage of Alzheimer’s disease. The drug had bagged FDA’s accelerated approval in January this year.
Similarly, Regeneron (-3 percent) was down on news that FDA has rejected a higher-dose variant of its blockbuster drug Eylea for the treatment of wet age-related macular degeneration (AMD), along with two other eye conditions common in diabetic patients. And Sarepta (-8 percent) was hit by news that analysts have voiced concerns over upcoming confirmatory trial data for its gene therapy — Elevidys — to treat Duchenne muscular dystrophy (DMD). While Elevidys has received an accelerated approval from the FDA as the first-of-its-kind gene therapy for DMD in children, analysts said the trial may not be enough to secure approval for the drug’s expanded use.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
Our view
Pharma stocks have had a tumultuous ride over the past year. While the Nasdaq Composite Index and S&P 500 have been going up, pharma indices have not seen such a rally due to reasons such as a drop in sales of Covid treatments, the loss of patent protections, and a looming recession.
In stock market parlance, this means pharma stocks may be undervalued and are likely to draw investor interest. Pathbreaking research and new drug pipelines should only increase their interest.
Access the Pipeline Prospector Dashboard for June 2023 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in June 2023
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers in June 2023 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







