
1. Hbr Of Trimethylamine
2. Hcl Of Trimethylamine
3. Hi Of Trimethylamine
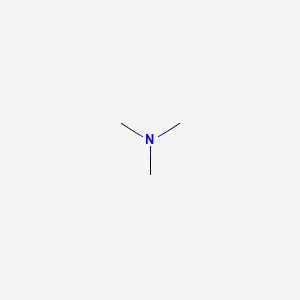
1. N,n-dimethylmethanamine
2. 75-50-3
3. Methanamine, N,n-dimethyl-
4. N-trimethylamine
5. Dimethylmethaneamine
6. Trimethylamine Solution
7. (ch3)3n
8. Trimethyl Amine
9. Fema No. 3241
10. Trimethylamine Anhydrous
11. Trimethyl-amine
12. Un1083
13. Un1297
14. N,n,n-trimethylamine
15. Nme3
16. Ai3-15639
17. Trimethylamine, In Aqueous Solution
18. Trimethylamine Solution (30% Or Less)
19. Methylamine, N,n-dimethyl-
20. Lhh7g8o305
21. Chebi:18139
22. Mfcd00008327
23. Trimethylamine, Anhydrous [un1083] [flammable Gas]
24. Trimethylamin
25. Fema Number 3241
26. Trimethylamine, Aqueous Solutions Not >50% Trimethylamine, By Mass [un1297] [flammable Liquid]
27. Trimethylamine Solution, 25 Wt. % In H2o
28. Ccris 6283
29. Hsdb 808
30. Einecs 200-875-0
31. Unii-lhh7g8o305
32. Tri-methylamine
33. Ken
34. Dimethylamino Methane
35. Trimethylaminum
36. Tridimethylaminomethane
37. Trimethylamine, Anhydrous
38. N,n-dimethyl-methanamine
39. Dsstox_cid_6238
40. N,n-dimethylmethanamine #
41. Bmse000224
42. Ec 200-875-0
43. Trimethylamine [mi]
44. Dsstox_rid_78071
45. Nciopen2_007868
46. Trimethylamine [fcc]
47. Dsstox_gsid_26238
48. Dimethylaminomethylidyneradical
49. Trimethylamine [fhfi]
50. Trimethylamine [hsdb]
51. Trimethylamine, >=99.0%
52. Trimethylamine, >=99.5%
53. Trimethylamine 2.0m In Thf
54. Trimethylaminum [hpus]
55. Trimethylamine Aqueous Solution
56. Chembl439723
57. Gtpl5521
58. Dtxsid2026238
59. Trimethylamine 2m In Isopropanol
60. N(ch3)3
61. Trimethylamine Solution, 34-36%
62. Trimethylamine, Anhydrous, >=99%
63. Zinc8216125
64. Tox21_302355
65. Trimethylamine, 1m Solution In Thf
66. Bdbm50416499
67. Nsc101179
68. Stl264242
69. Akos000119986
70. Trimethylamine 2m Solution In Methanol
71. Trimethylamine Solution 20 % In H2o
72. Zinc112948558
73. Nsc-101179
74. Un 1083
75. Un 1297
76. Cas-75-50-3
77. Ncgc00255170-01
78. Trimethylamine, 45% W/w Aqueous Solution
79. Ft-0660006
80. T0464
81. T2268
82. T2704
83. T2892
84. T2893
85. T3567
86. T3614
87. T3847
88. C00565
89. Trimethylamine (ca.8% In N,n-dimethylformamide)
90. Trimethylamine Solution, 25 Wt. % In H2o, Fg
91. Trimethylamine Solution, 45 Wt. % In H2o, Fg
92. Q423953
93. Trimethylamine (ca. 8% In Toluene, Ca. 1mol/l)
94. Trimethylamine Solution, ~45 Wt. % In H2o (t)
95. Meldonium Dihydrate Impurity A [ep Impurity]
96. Acetylcholine Chloride Impurity C [ep Impurity]
97. Trimethylamine Solution, 25 Wt. % In Propylene Glycol
98. F1908-0091
99. Trimethylamine (~25 Wt. Per Cent Solution In Methanol)
100. Trimethylamine (ca. 13% In Acetonitrile, Ca. 2mol/l)
101. Trimethylamine (ca. 25% In Isopropyl Alcohol, Ca. 3mol/l)
102. Trimethylamine Solution (ca. 28% In Water, Ca. 4.3mol/l)
103. Trimethylamine Solution (ca. 25% In Isopropyl Alcohol, Ca. 3mol/l)
104. Trimethylamine, Anhydrous, Cylinder, With 316ss Needle Valve, 99%
105. Trimethylamine, Aqueous Solutions Not >50% Trimethylamine, By Mass
106. Hydrogen Undecahydrodicarbaundecaborate(1-), Compd. With Trimethylamine (1:1)
107. Trimethylamine Solution, 31-35 Wt. % In Ethanol, 4.2 M, Contains Toluene As Stabilizer
| Molecular Weight | 59.11 g/mol |
|---|---|
| Molecular Formula | C3H9N |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 3.2 |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The absorption, distribution, and biotransformation of TMA ... /was/ followed in rats given intravenous doses of 1 to 2 g/kg. TMA was rapidly distributed to tissues, especially the liver. In rats, TMA was 81% available after oral administration, reached a peak blood level 1 hour after oral administration, and was cleared with a half-life of 2 to 2.5 hours. When fed a synthetic diet, clearance showed a two-fold reduction. Conversion of TMA to its metabolite, trimethylamine-N-oxide, proceeded slowly in liver homogenates. In humans, TMA is formed in the intestinal tract from dietary choline. Large doses of choline result in disproportionately higher formation and urinary excretion of TMA. In newborn dairy calves, fecal trimethylamine levels are clearly higher in milk-fed calves and show huge elevations in diarrheic cases. Although fish is a major source of TMA in the diet, strawberries, kale juice, and garlic have been shown to increase urinary TMA levels.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2
TMA penetrated both rat and human skin readily when applied to the epidermal surface with flux rates ranging from 3.4 to 265 ug/sq cm/hour in rats and from 0.98 to 92.7 ug/sq cm/hour in humans with dose applications of 0.1, 1, and 10 mg per 0.32 sq cm skin membrane. Both rat and human skin could act as a reservoir and evidence exists for small but detectable N-oxide forming during passage through the skin.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2
... About 50% of the dose of trimethylamine hydrochloride (administered orally to dogs) was eliminated unchanged together with traces of dimethylamine, suggesting that trimethylamine was N-dealkylated.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 713
It can be absorped through the skin and/or eye contact, inhalation, and ingestion (solution).
Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens, 2002. 4th ed.Vol 1 A-H Norwich, NY: Noyes Publications, 2002., p. 2275
For more Absorption, Distribution and Excretion (Complete) data for Trimethylamine (6 total), please visit the HSDB record page.
When administered to humans, dogs, or rabbits, /trimethylamine/ is partly degraded to ammonia and subsequently to urea, and oxidized to trimethylamine oxide.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V4 713
The Sperber technique of infusion into the renal portal circulation in chickens was used to investigate in vivo the renal tubular transport and renal metabolism of trimethylamine (TMA). When 14C-TMA was infused at a rate of 1 x 10(-9) mol/min the transport efficiency (TE), that is, the tubular excretion of the (14)C-label relative to excretion of simultaneously infused paminohippuric acid, was 0.70. Progressive addition of unlabeled TMA up to infusion rates of 1 x 10(-5) mol/min produced a progressive fall in the TE of the (14)C-label. Identification of the (14)C-label excreted in the urine revealed that approximately 85% of the infused (14)C-TMA was excreted by the infused kidney as a single metabolite over the entire range of infusions. By use of the techniques of low-voltage electrophoresis, high-voltage electrophoretic mobility-pH profile, and gas chromatography/mass spectrometry, the renal metabolite was found to be identical with standard (14)C-trimethylamine oxide (TMAO). At a TMA infusion rate of 1.5 x 10(-6) mol/kg/min reaching the infused kidney, the rate at which TMAO was formed and excreted by the kidney was 0.12 x 10(-6) mol per g of kidney per min. When (14)C-TMAO was infused into chickens its TE was 0.11, which was not evidence for active excretory transport. Infused TMA was almost entirely metabolized in vivo to its N-oxide, TMAO, which then entered the urine. The renal tubular excretion of (14)C during infusion of (14)C-TMA was inhibited by the cationic blocker of transport, quinine, and by the anionic blocker of transport, probenecid.
PMID:13980 Acara M et al; Drug Metab Dispos 5 (1): 82-90 (1977)
Humans ingest substantial amounts of choline and lecithin as part of common foods. Physicians have recently begun administering large doses of these compounds to individuals with neurological diseases. A significant fraction of ingested choline is destroyed by enzymes within gut bacteria, forming trimethylamine (TMA), dimethylamine (DMA) and monomethylamine (MMA). Some of these methylamines are eventually excreted into the urine, presumably after being absorbed and carried to the kidneys via the bloodstream. The methylamines formed after choline is eaten could be substrates for the formation of nitrosamines, which have marked carcinogenic activity. Twenty-seven millimoles of choline chloride, choline stearate or lecithin were administered to healthy human subjects. It was found that these treatments markedly increased the urinary excretion of TMA, DMA and MMA, with choline chloride having the greatest effect. Rats were treated with 2 mmol/kg b.wt. of choline chloride or lecithin, and it was found that these treatments significantly increased urinary TMA excretion and did not alter DMA or MMA excretion. Our choline chloride preparation contained no MMA, DMA or TMA; however, it was found that our choline stearate and all the commercially available lecithins tested were contaminated with methylamines. Prior removal of methylamines from our lecithin preparation minimized the effect of oral administration of this compound on methylamine excretion in urine of rats and humans.
PMID:6842395 Zeisel SH et al; J Pharmacol Exp Ther 225 (2): 320-4 (1983)
Flavin-containing monooxygenase form 3 (FMO3) is one of the major enzyme systems that protect humans from the potentially toxic properties of drugs and chemicals. Flavin-containing monooxygenase form 3 converts nucleophilic heteroatom-containing chemicals and endogenous materials to polar metabolites, which facilitates their elimination. For example, the tertiary amine trimethylamine is N-oxygenated by human flavin-containing monooxygenase form 3 to trimethylamine N-oxide, and trimethylamine N-oxide is excreted in a detoxication and deoderation process. In normal humans, virtually all trimethylamine is metabolized to trimethylamine N-oxide. In a few humans, trimethylamine is not efficiently metabolized to trimethylamine N-oxide, and those individuals suffer from trimethylaminuria, or fishlike odor syndrome. Previously ... mutations of the flavin-containing monooxygenase form 3 gene that cause trimethylaminuria /were identified/. /The authors/ report two prevalent polymorphisms of this gene (K158E and V257M) that modulate the activity of human monooxygenase form 3.
PMID:10640514 Cashman JR et al; Drug Metab Dispos 28 (2): 169-73 (2000)
For more Metabolism/Metabolites (Complete) data for Trimethylamine (10 total), please visit the HSDB record page.
Uremic toxins tend to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces.
In rats, TMA was 81% available after oral administration, reached a peak blood level 1 hour after oral administration, and was cleared with a half-life of 2 to 2.5 hours.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 2

