
1. 172796-84-8
2. Schembl18107090
3. Dtxsid70714748
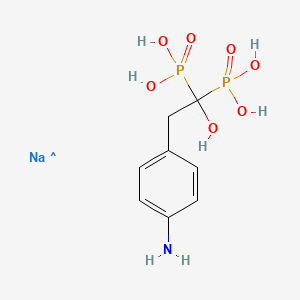
| Molecular Weight | 320.13 g/mol |
|---|---|
| Molecular Formula | C8H13NNaO7P2 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 161 |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 359 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
BUILDING BLOCK

CAS Number : 172796-84-8
End Use API :
End Use API : Tetraethyl methylenediphosphonate
About the Company : JPN Pharma is a premier pharmaceutical company in India, specializing in the development and manufacturing of Active Pharmaceutical Ingredients (APIs) and drug intermediates. Headquarte...
