
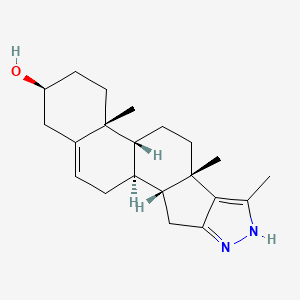

1. Zinc3983817
| Molecular Weight | 326.5 g/mol |
|---|---|
| Molecular Formula | C21H30N2O |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 326.235813585 g/mol |
| Monoisotopic Mass | 326.235813585 g/mol |
| Topological Polar Surface Area | 48.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
BUILDING BLOCK


