NDC Code(s) : 80830-1693-1, 80830-1693-5, 80830-1693-3, 80830-1691-1, 80830-1691-5, 80830-1691-2, 80830-1692-1, 80830-1692-5, 80830-1692-2
Packager : Puniska Healthcare Private Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| POTASSIUM PHOSPHATESpotassium phosphate, monobasic potassium phosphate, dibasic injection, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| POTASSIUM PHOSPHATESpotassium phosphate, monobasic potassium phosphate, dibasic injection, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| POTASSIUM PHOSPHATESpotassium phosphate, monobasic potassium phosphate, dibasic injection, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Puniska Healthcare Private Limited(675474666) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Puniska Healthcare Private Limited | 675474666 | analysis(80830-1693, 80830-1691, 80830-1692), label(80830-1693, 80830-1691, 80830-1692), manufacture(80830-1693, 80830-1691, 80830-1692), pack(80830-1693, 80830-1691, 80830-1692), sterilize(80830-1693, 80830-1691, 80830-1692) | |
PRINCIPAL DISPLAY PANEL
NDC 80830-1693-1
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 15 mmol/5 mL (3 mmol/mL)
Potassium 22 mEq/5 mL (4.4 mEq/mL)
5 mL Vial Label
Rx only
Amneal Pharmaceuticals LLC

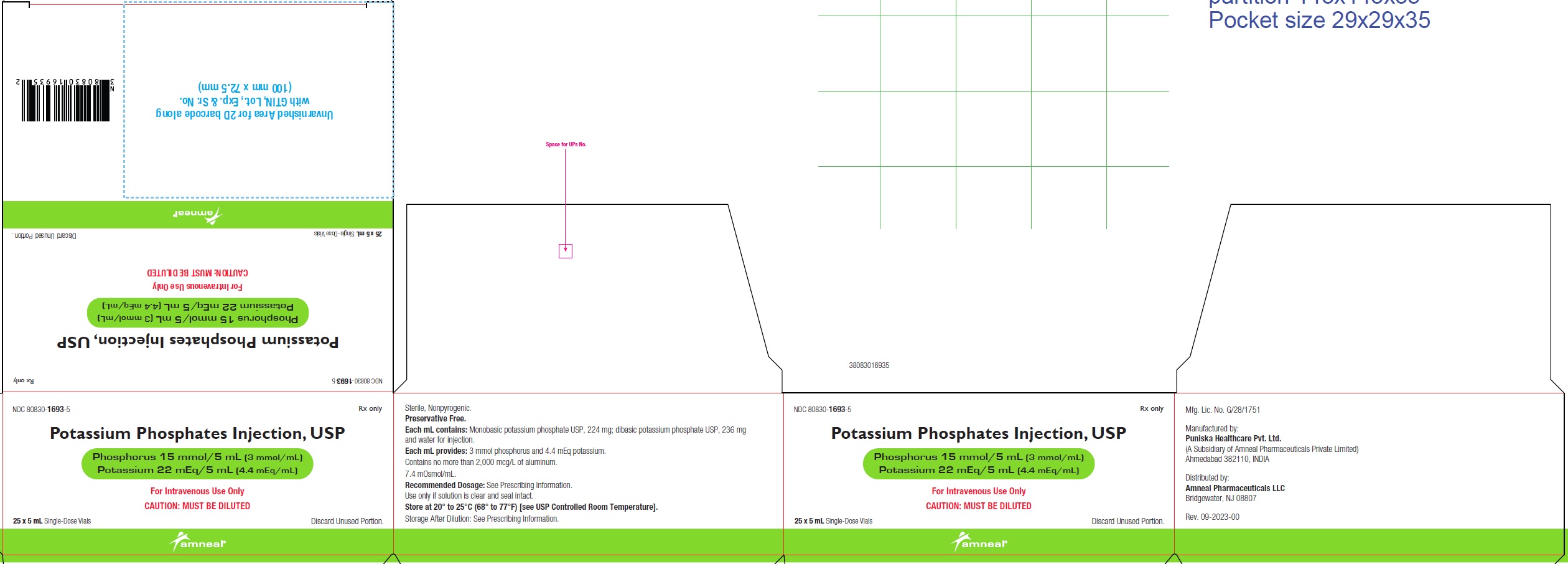
NDC 80830-1693-5
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 15 mmol/5 mL (3 mmol/mL)
Potassium 22 mEq/5 mL (4.4 mEq/mL)
25 x 5 mL Carton Label
Rx only
Amneal Pharmaceuticals LLC
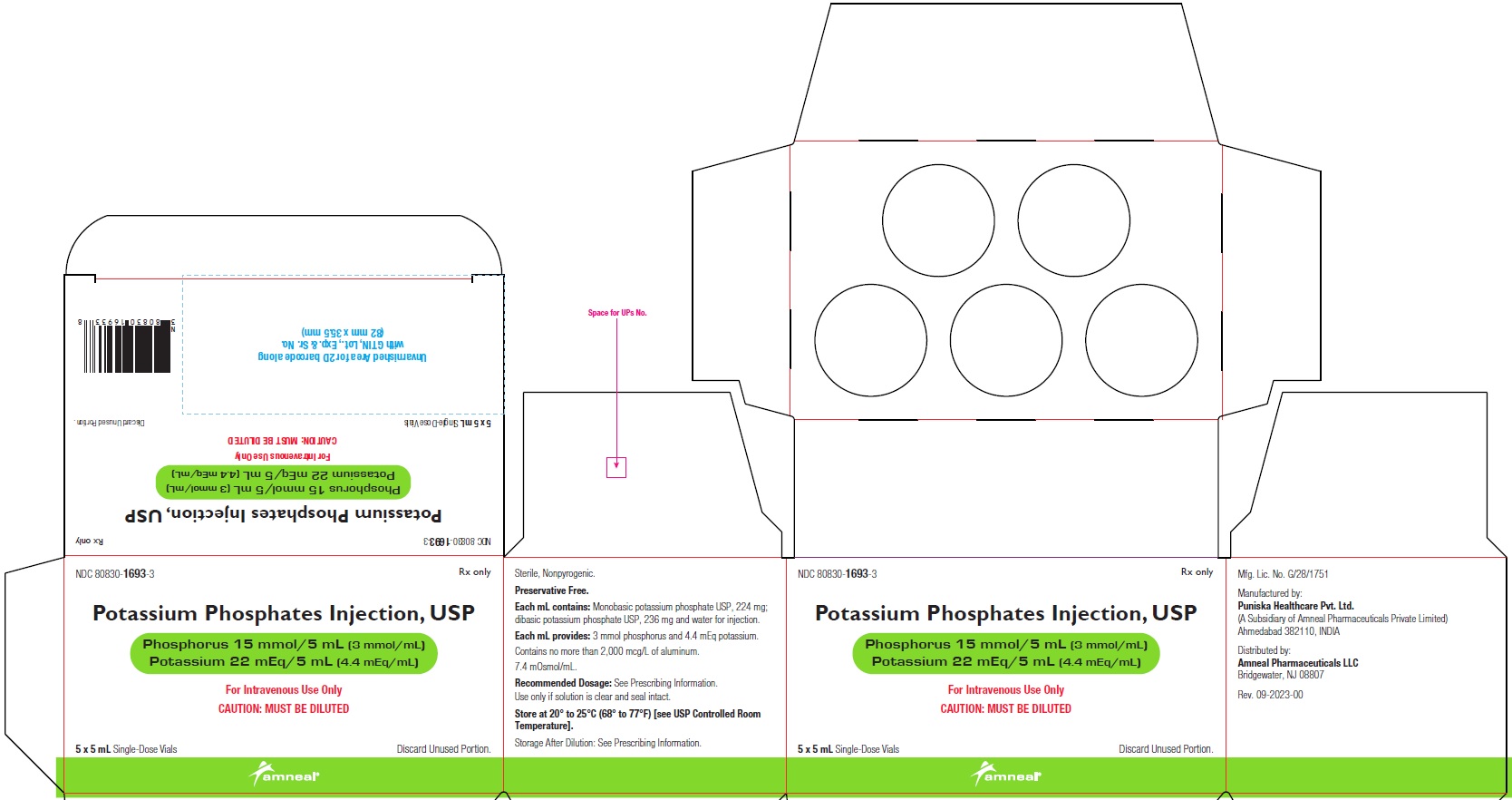
NDC 80830-1693-3
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 15 mmol/5 mL (3 mmol/mL)
Potassium 22 mEq/5 mL (4.4 mEq/mL)
5 x 5 mL Carton Label
Rx only
Amneal Pharmaceuticals LLC
NDC 80830-1691-1
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 45 mmol/15 mL (3 mmol/mL)
Potassium 66 mEq/15 mL (4.4 mEq/mL)
15 mL Vial Label
Rx only
Amneal Pharmaceuticals LLC

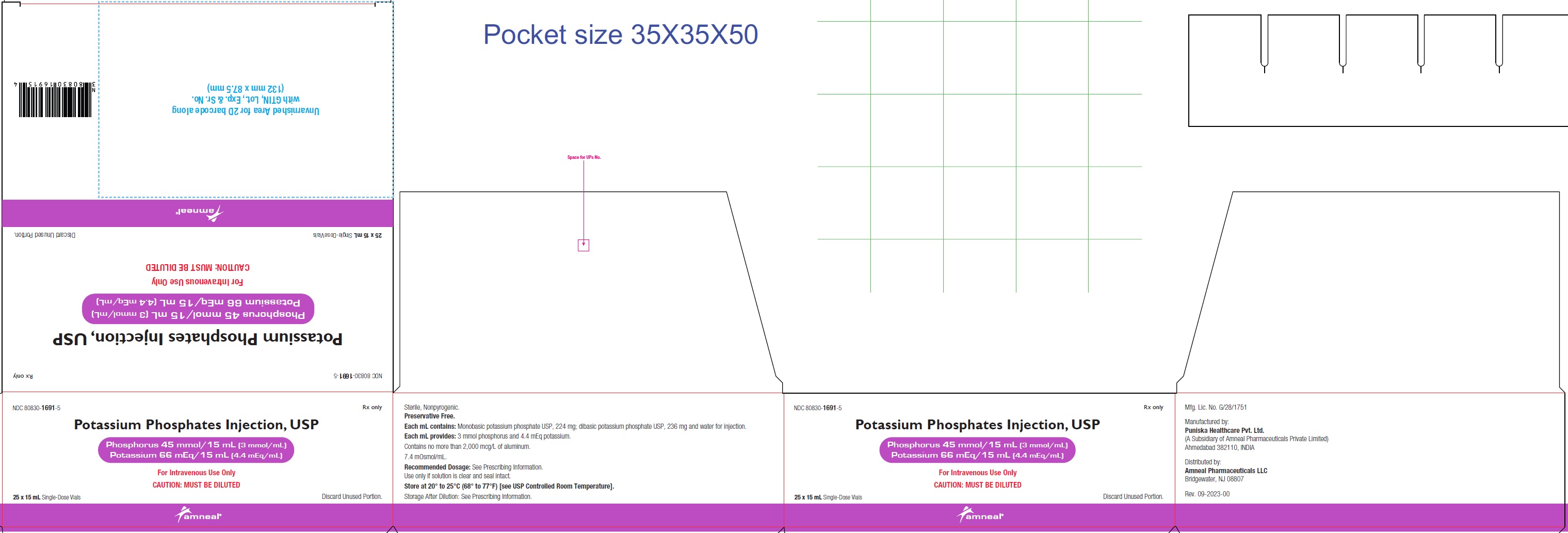
NDC 80830-1691-5
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 45 mmol/15 mL (3 mmol/mL)
Potassium 66 mEq/15 mL (4.4 mEq/mL)
25 x 1 5 mL Carton Label
Rx only
Amneal Pharmaceuticals LLC
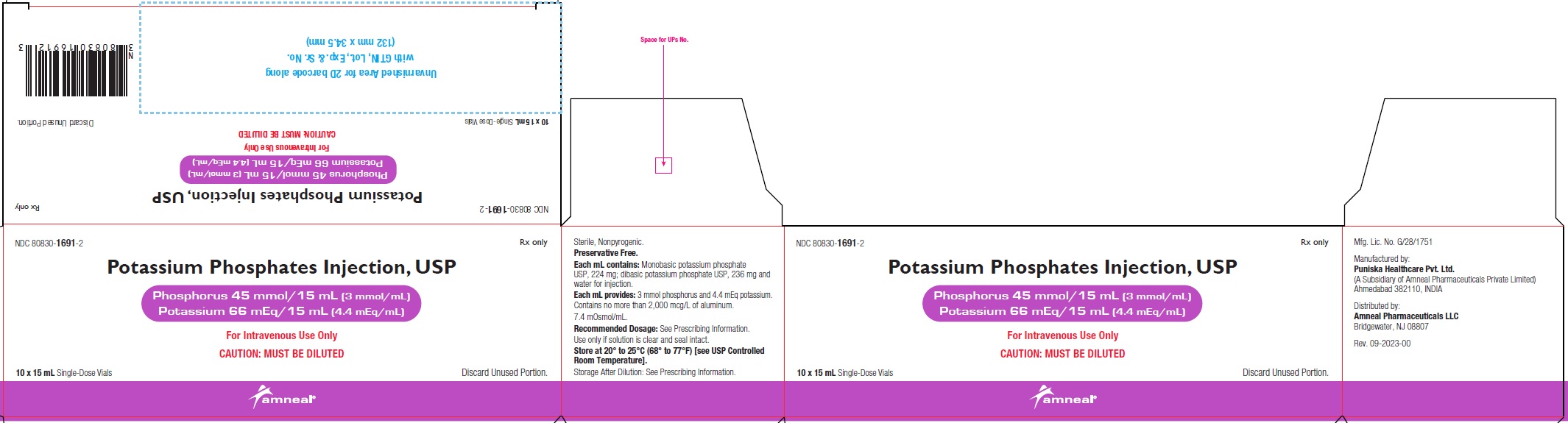
NDC 80830-1691-2
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 45 mmol/15 mL (3 mmol/mL)
Potassium 66 mEq/15 mL (4.4 mEq/mL)
10 x 15 mL Carton Label
Rx only
Amneal Pharmaceuticals LLC
NDC 80830-1692-1
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 150 mmol/50 mL (3 mmol/mL)
Potassium 220 mEq/50 mL (4.4 mEq/mL)
50 mL Pharmacy Bulk Package Vial Label
Rx only
Amneal Pharmaceuticals LLC

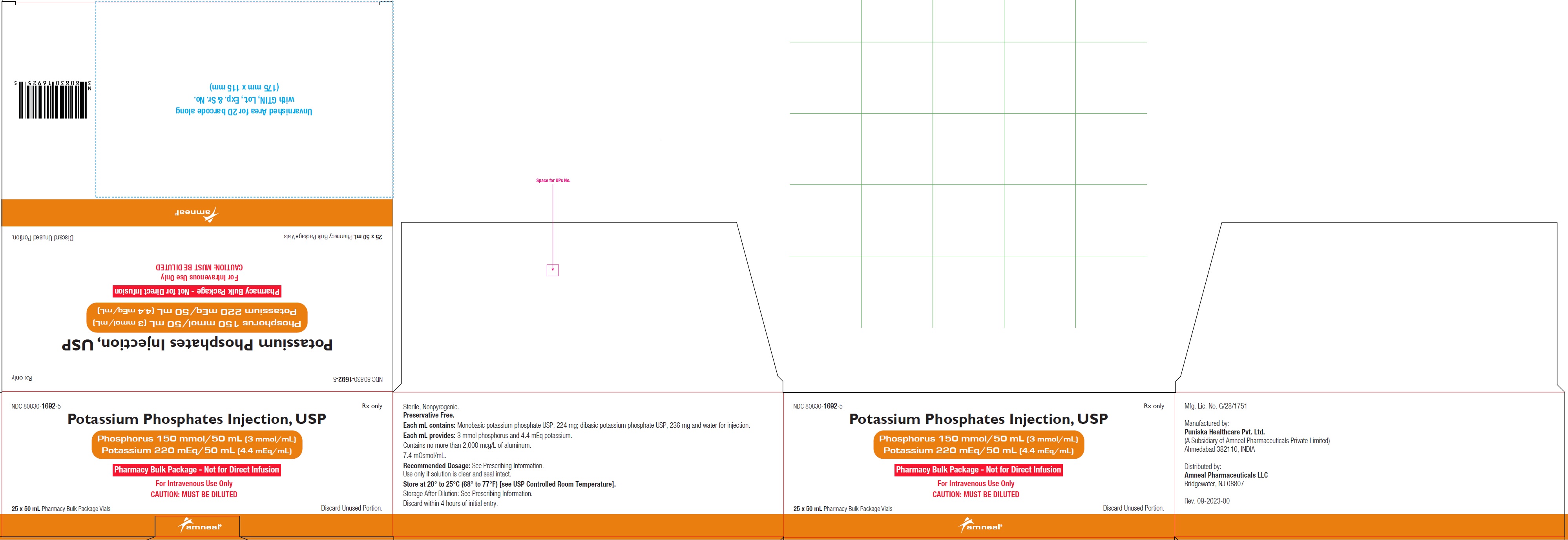
NDC 80830-1692-5
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 150 mmol/50 mL (3 mmol/mL)
Potassium 220 mEq/50 mL (4.4 mEq/mL)
25 x 50 mL Pharmacy Bulk Package Carton Label
Rx only
Amneal Pharmaceuticals LLC
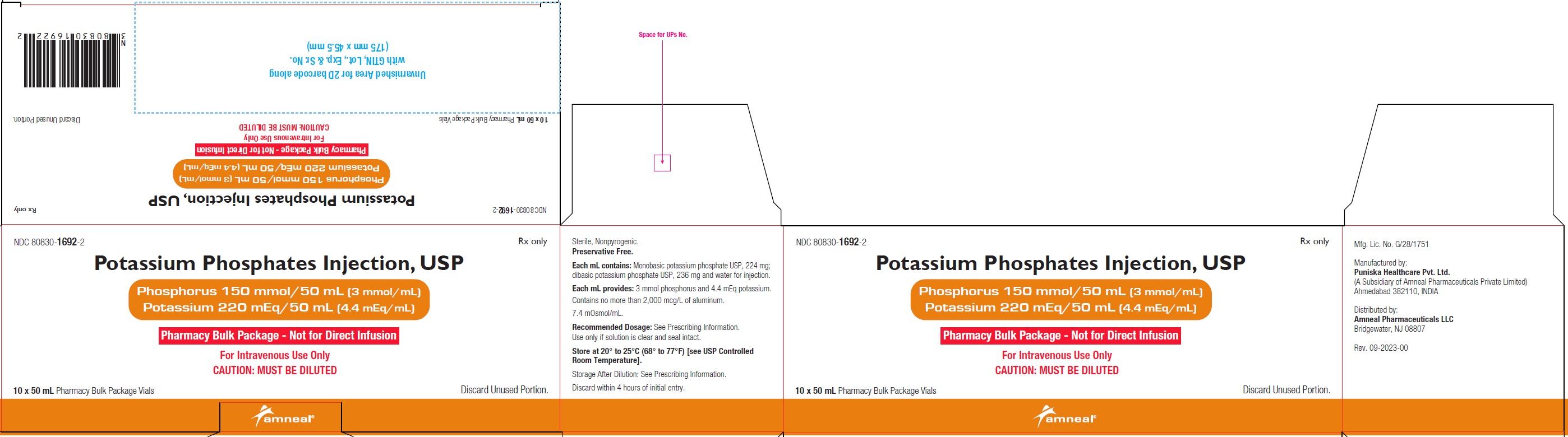
NDC 80830-1692-2
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 150 mmol/50 mL (3 mmol/mL)
Potassium 220 mEq/50 mL (4.4 mEq/mL)
10 x 50 mL Pharmacy Bulk Package Carton Label
Rx only
Amneal Pharmaceuticals LLC
POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 150 mmol/50 mL (3 mmol/mL)
Potassium 220 mEq/50 mL (4.4 mEq/mL)
25 x 50 mL Sticker Label (Pharmacy Bulk Package)
Amneal Pharmaceuticals LLC

POTASSIUM PHOSPHATES INJECTION, USP
Phosphorus 150 mmol/50 mL (3 mmol/mL)
Potassium 220 mEq/50 mL (4.4 mEq/mL)
10 x 50 mL Sticker Label (Pharmacy Bulk Package)
Amneal Pharmaceuticals LLC