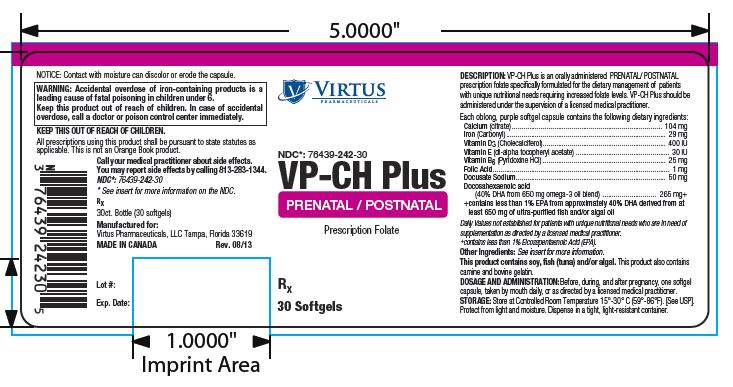
NDC Code(s) : 76439-242-30
Packager : Virtus Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VP CH Pluscalcium citrate, iron pentacarbonyl, cholecalciferol, .alpha.-tocopherol acetate, dl-, pyridoxine hydrochloride, folic acid, docusate sodium and doconexent CAPSULE, GELATIN COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Virtus
PHARMACEUTICALS
NDC: 76439-242-30
VP-CH Plus
PRENATAL / POSTNATAL
Prescription Folate
Rx
30 Softgels