NDC Code(s) : 72888-034-30, 72888-034-01, 72888-034-05, 72888-034-00, 72888-035-30, 72888-035-01, 72888-035-05, 72888-035-00, 72888-036-30, 72888-036-01, 72888-036-05, 72888-036-00, 72888-037-30, 72888-037-01, 72888-037-05, 72888-037-00
Packager : Advagen Pharma Ltd
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CARVEDILOLCARVEDILOL TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARVEDILOLCARVEDILOL TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARVEDILOLCARVEDILOL TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CARVEDILOLCARVEDILOL TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Advagen Pharma Ltd(051627256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Rubicon Research Private Limited | 677604197 | manufacture(72888-034, 72888-035, 72888-036, 72888-037), analysis(72888-034, 72888-035, 72888-036, 72888-037), pack(72888-034, 72888-035, 72888-036, 72888-037) | |
PRINCIPAL DISPLAY PANEL
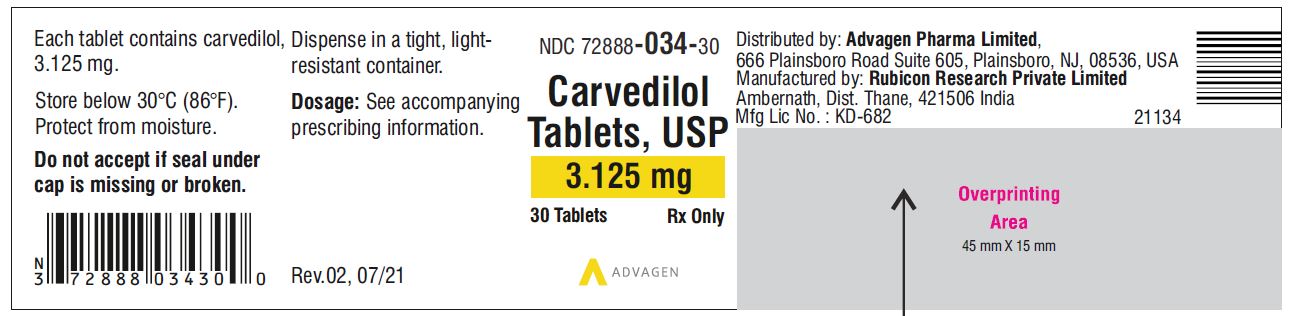
Carvedilol Tablets USP, 3.125 mg - NDC 72888-034-30 - 30 Tablets Container Label
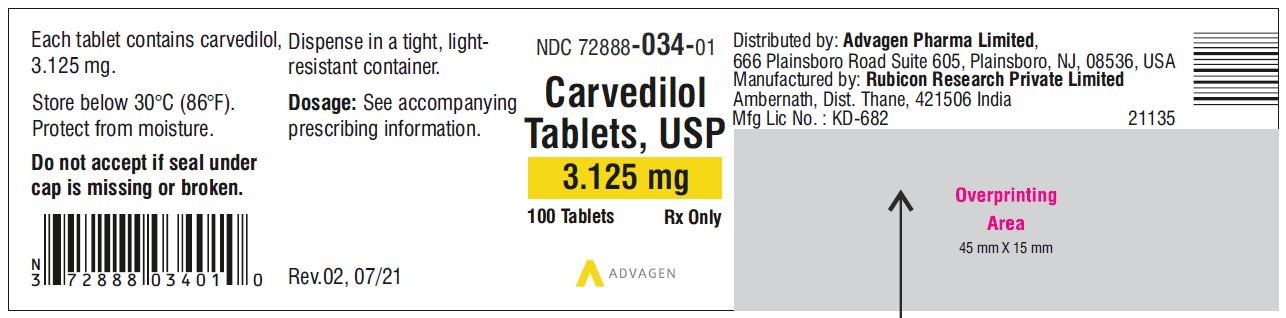
Carvedilol Tablets USP, 3.125 mg - NDC 72888-034-01 - 100 Tablets Container Label
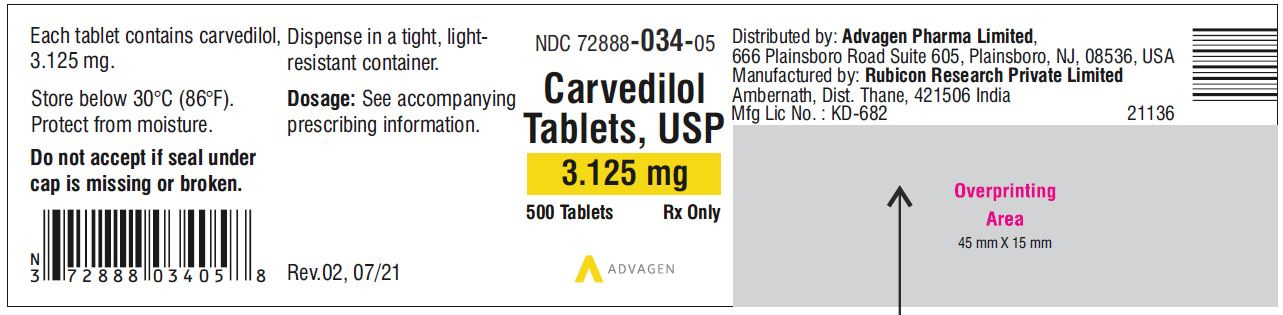
Carvedilol Tablets USP, 3.125 mg - NDC 72888-034-05 - 500 Tablets Container Label
Carvedilol Tablets USP, 3.125 mg - NDC 72888-034-00 - 1000 Tablets Container Label
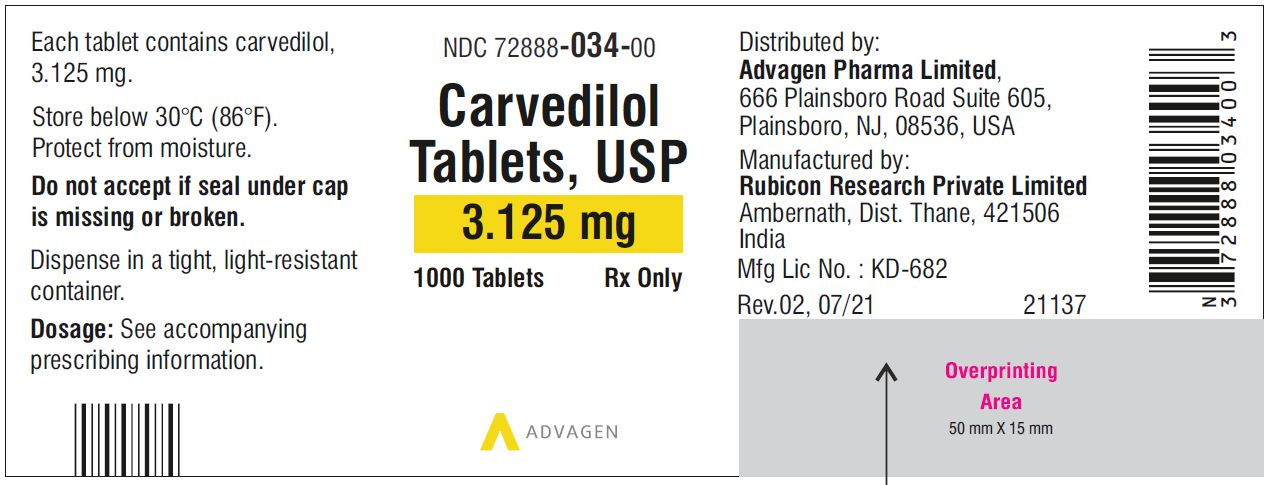
Carvedilol Tablets USP, 6.25 mg - NDC 72888-035-30 - 30 Tablets Container Label
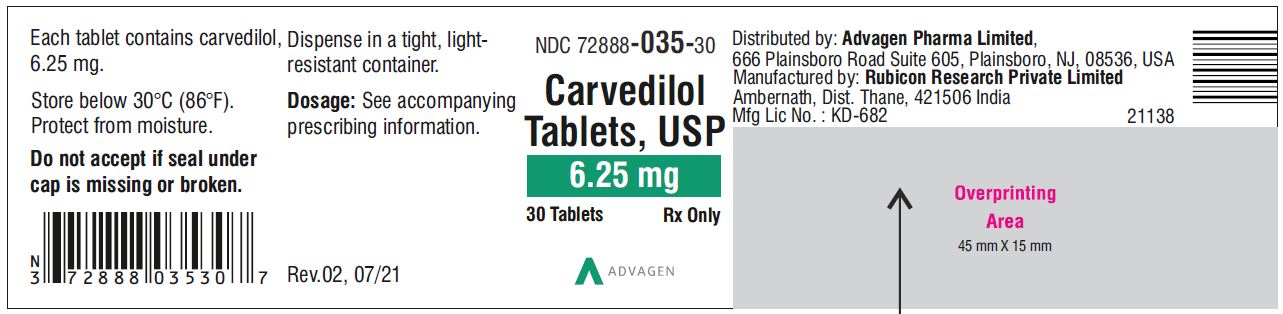
Carvedilol Tablets USP,6.25 mg - NDC 72888-035-01 - 100 Tablets Container Label
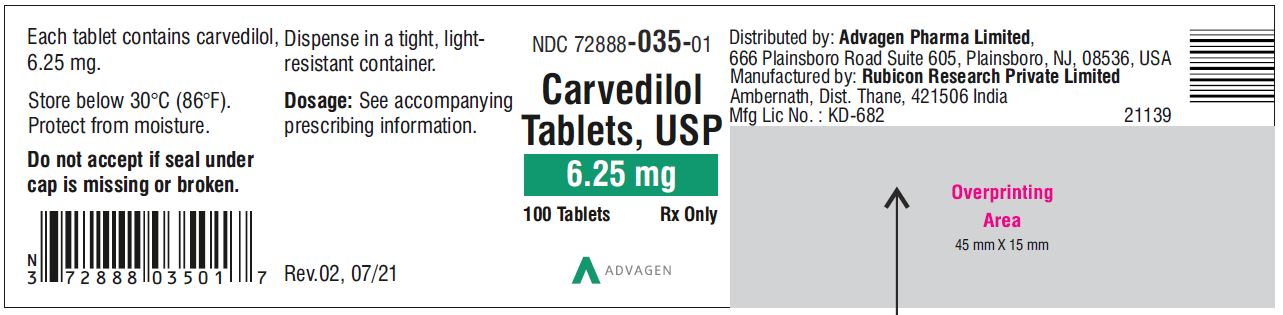
Carvedilol Tablets USP, 6.25 mg - NDC 72888-035-05 - 500 Tablets Container Label
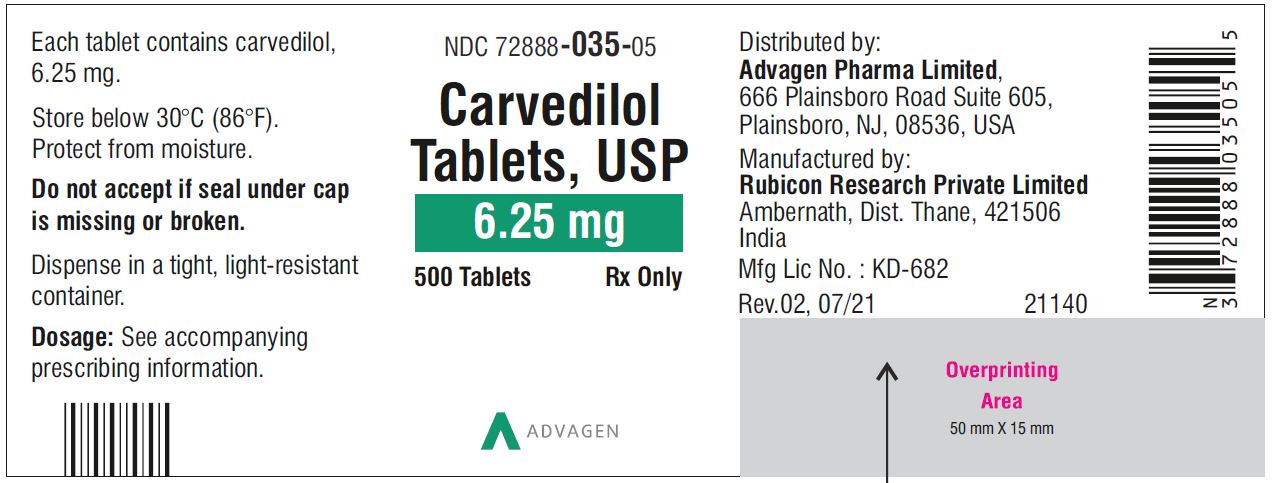
Carvedilol Tablets USP, 6.25 mg - NDC 72888-035-00 - 1000 Tablets Container Label
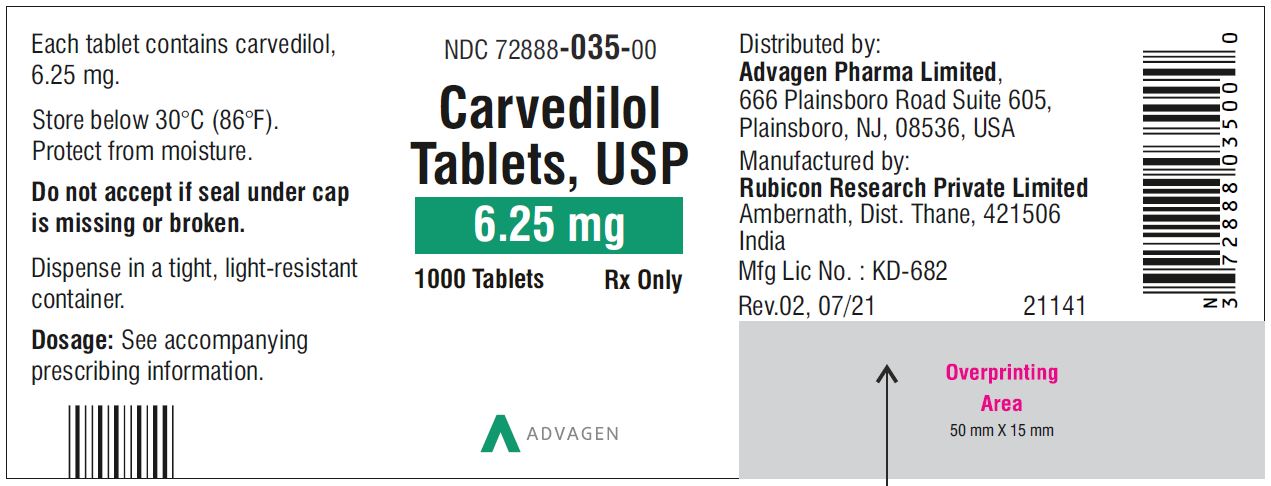
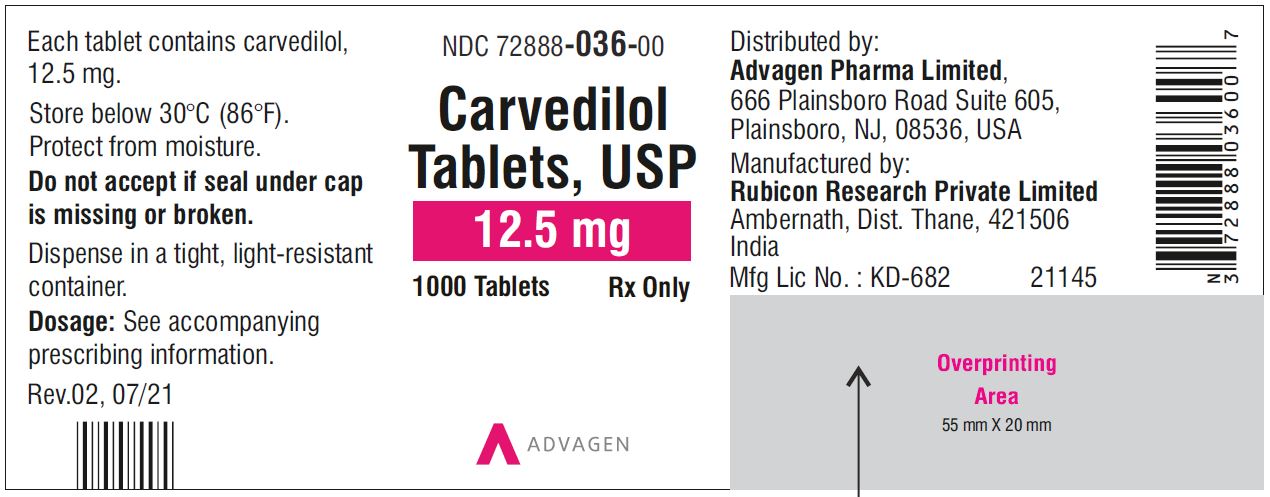
Carvedilol Tablets USP, 12.5 mg - NDC 72888-036-30 - 30 Tablets Container Label
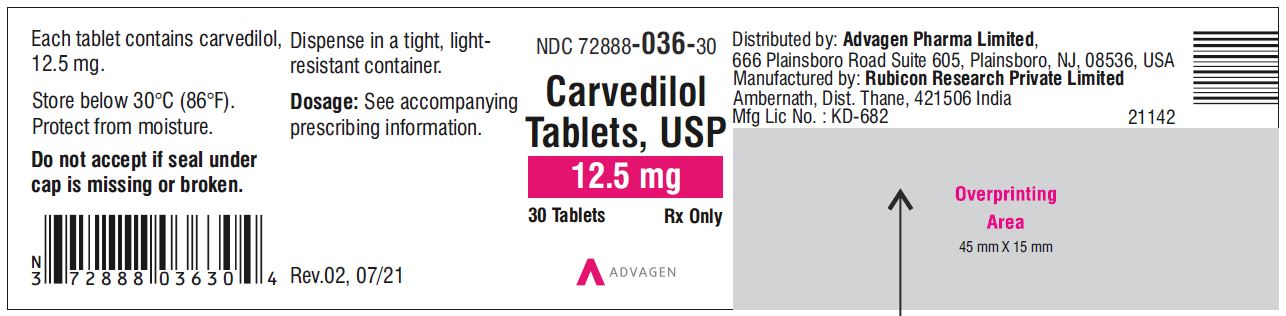
Carvedilol Tablets USP, 12.5 mg - NDC 72888-036-01 - 100 Tablets Container Label
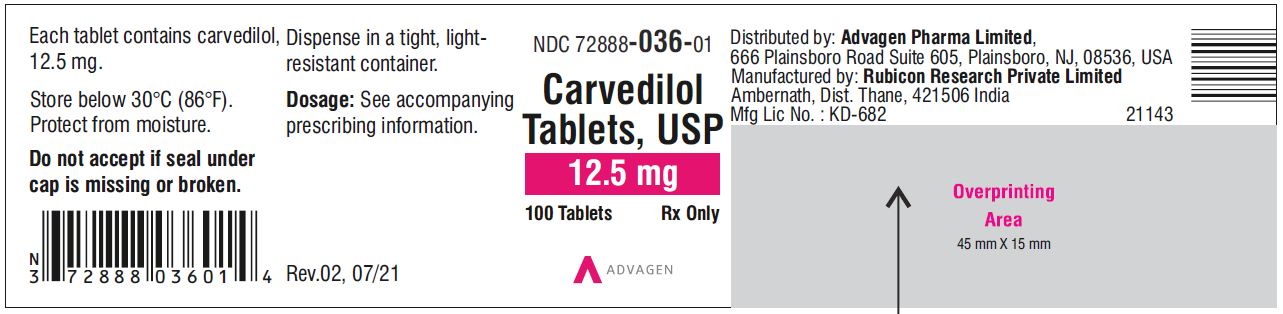
Carvedilol Tablets USP, 12.5 mg - NDC 72888-036-05 - 500 Tablets Container Label
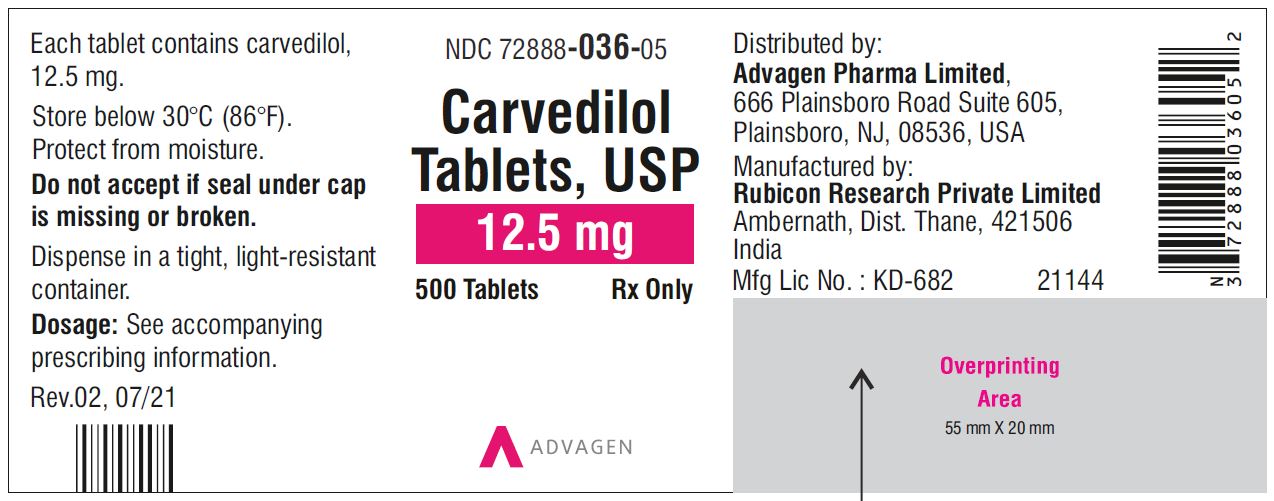
Carvedilol Tablets USP, 12.5 mg - NDC 72888-036-00 - 1000 Tablets Container Label
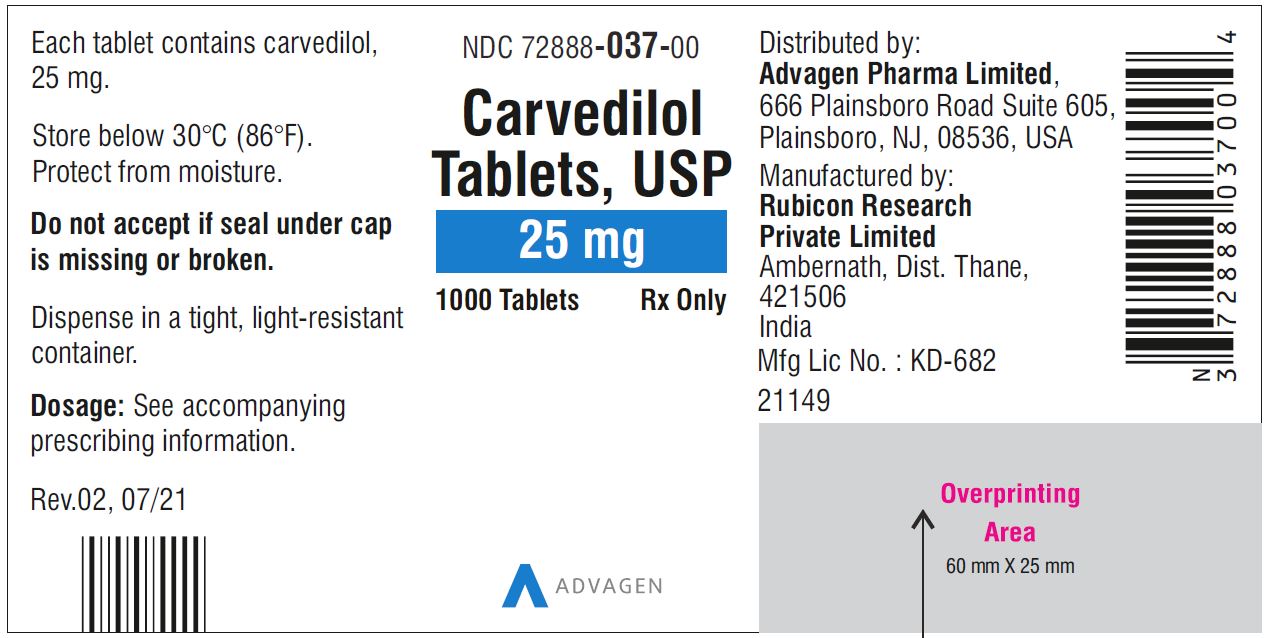
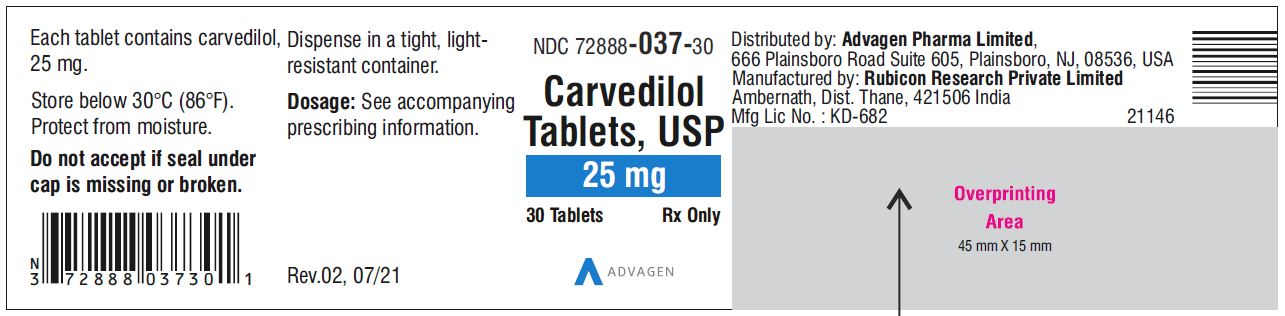
Carvedilol Tablets USP, 25 mg - NDC 72888-037-30 - 30 Tablets Container Label
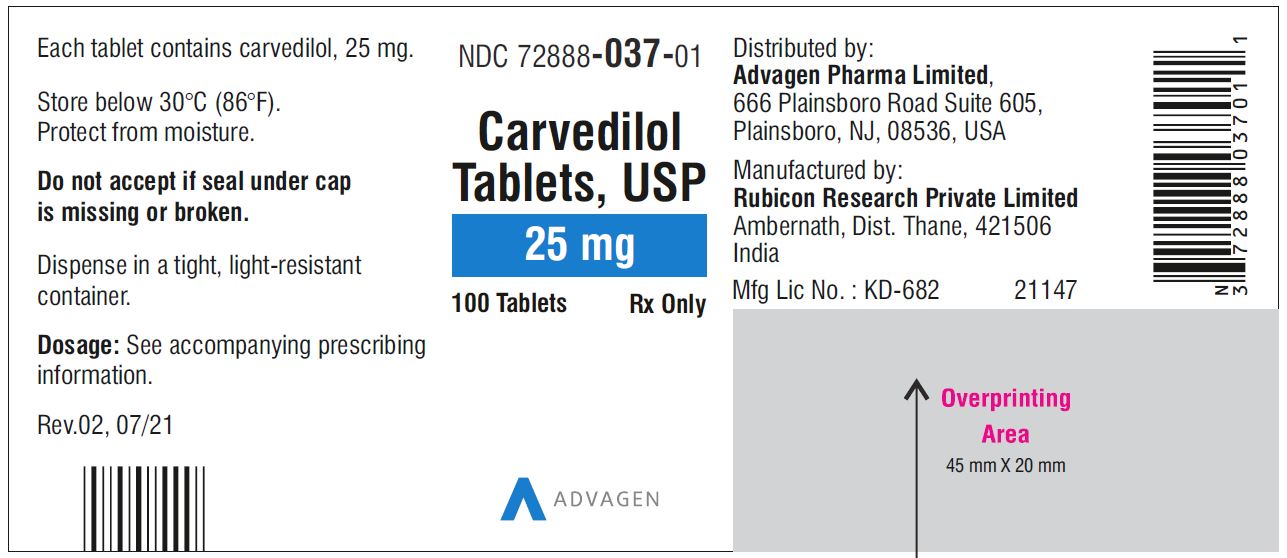
Carvedilol Tablets USP, 25 mg - NDC 72888-037-01 - 100 Tablets Container Label
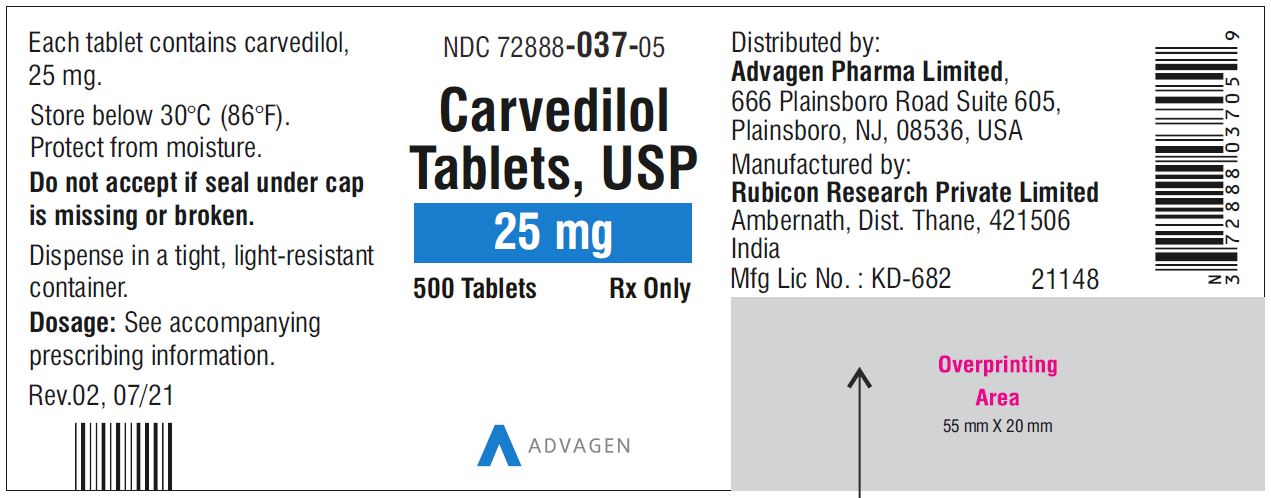
Carvedilol Tablets USP, 25 mg - NDC 72888-037-05 - 500 Tablets Container Label
Carvedilol Tablets USP, 25 mg - NDC 72888-037-00 - 1000 Tablets Container Label