NDC Code(s) : 71839-109-10, 71839-110-10, 71839-111-10, 71839-112-10, 71839-113-10, 71839-115-10, 71839-116-10
Packager : BE Pharmaceuticals Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| enoxaparin sodium enoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - BE Pharmaceuticals Inc.(081499296) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Biological E. Limited | 871410645 | ANALYSIS(71839-109, 71839-110, 71839-111, 71839-112, 71839-113, 71839-115, 71839-116), MANUFACTURE(71839-109, 71839-110, 71839-111, 71839-112, 71839-113, 71839-115, 71839-116) | |
PRINCIPAL DISPLAY PANEL
Enoxaparin Sodium Injection USP, 100 mg/mL (30 mg/0.3 mL) - PFS Label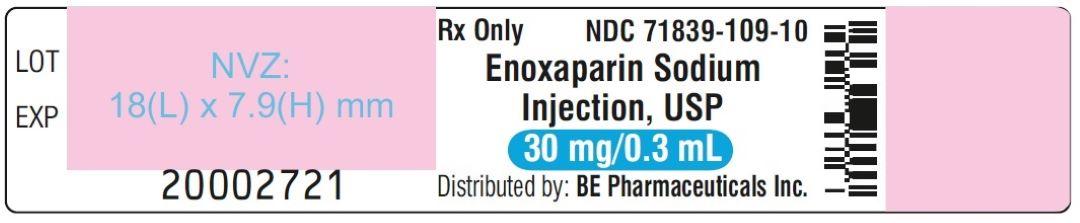
Enoxaparin Sodium Injection USP, 100 mg/mL (40 mg/0.4 mL) - PFS Label
Enoxaparin Sodium Injection USP, 100 mg/mL (60 mg/0.6mL) - PFS Label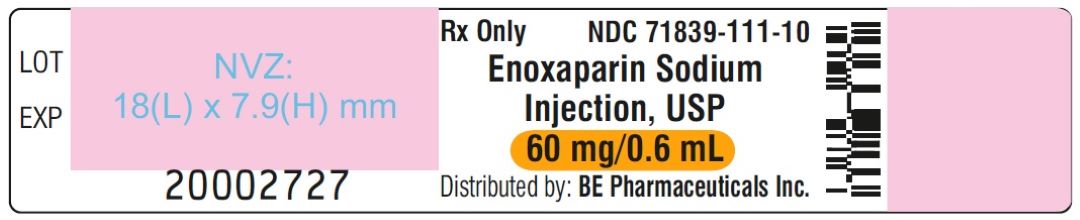
Enoxaparin Sodium Injection USP, 100 mg/mL (80 mg/0.8 mL) - PFS Label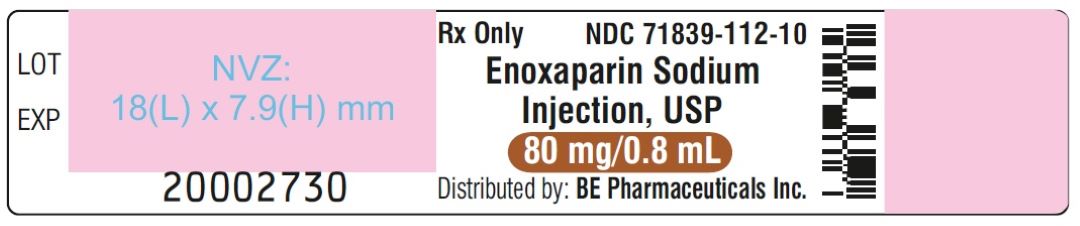
Enoxaparin Sodium Injection USP, 100 mg/mL (100 mg/1 mL) - PFS Label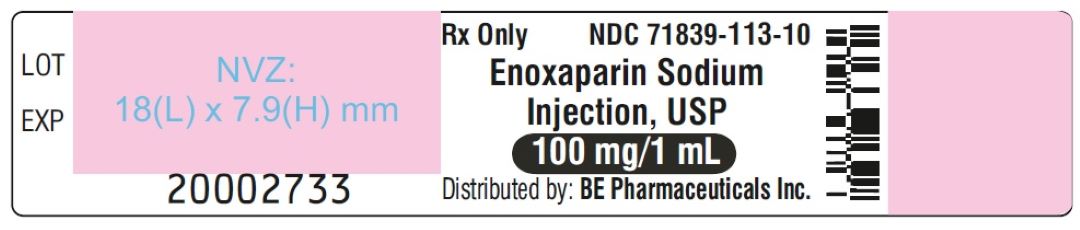
Enoxaparin Sodium Injection USP, 150 mg/mL (120 mg/0.8 mL) - PFS Label
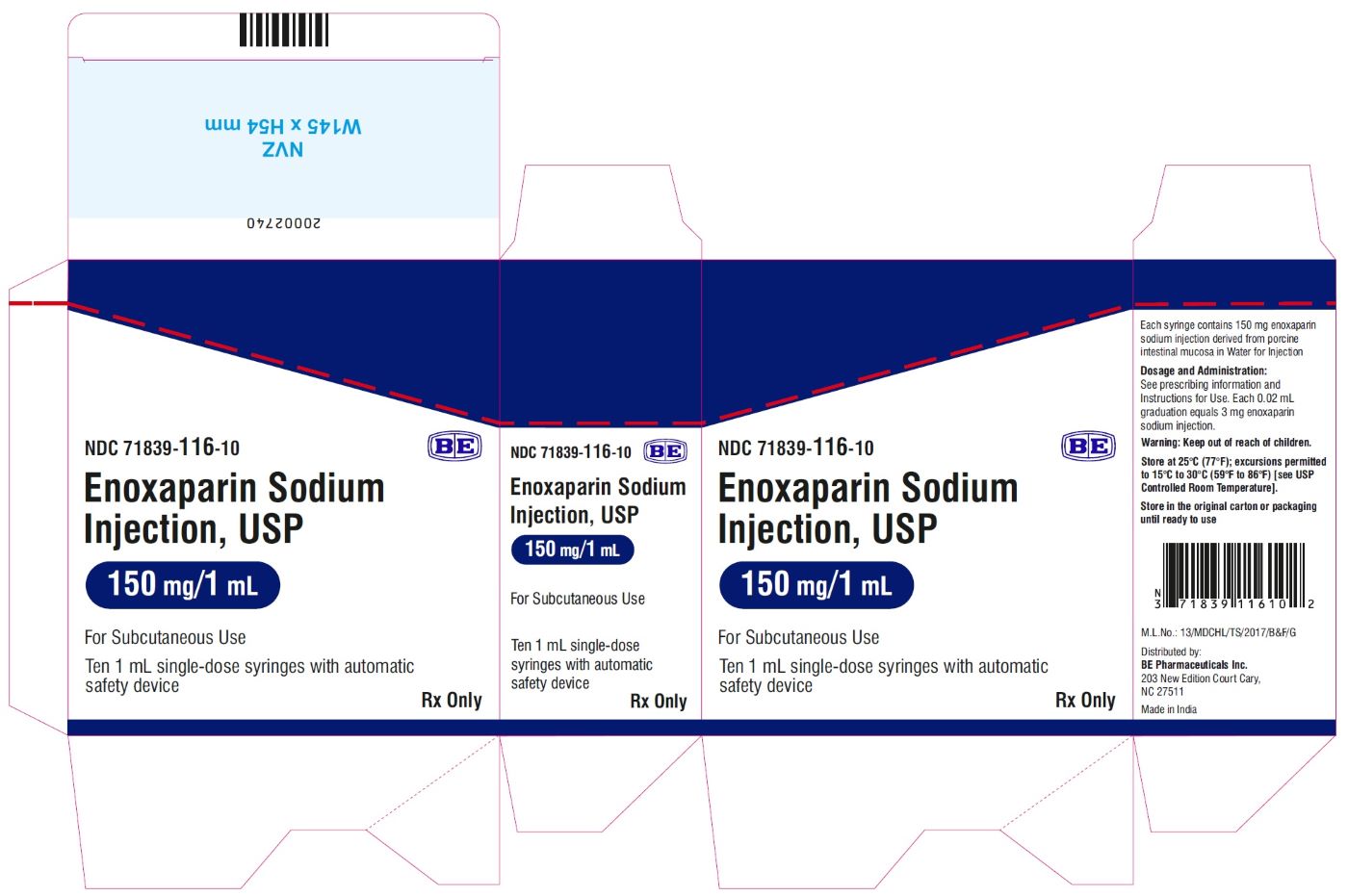
Enoxaparin Sodium Injection USP, 150 mg/mL (150 mg/1 mL) - PFS Label

Enoxaparin Sodium Injection USP, 100 mg/mL (30 mg/0.3 mL) - Blister Label

Enoxaparin Sodium Injection USP, 100 mg/mL (40 mg/0.4 mL) - Blister Label
Enoxaparin Sodium Injection USP, 100 mg/mL (60 mg/0.6 mL) - Blister Label

Enoxaparin Sodium Injection USP, 100 mg/mL (80 mg/0.8 mL) - Blister Label

Enoxaparin Sodium Injection USP, 100 mg/mL (100 mg/1 mL) - Blister Label

Enoxaparin Sodium Injection USP, 150 mg/mL (120 mg/0.8 mL) - Blister Label
Enoxaparin Sodium Injection USP, 150 mg/mL (150 mg/1 mL) - Blister Label
Enoxaparin Sodium Injection USP, 100 mg/mL (30 mg/0.3 mL) - Carton Label
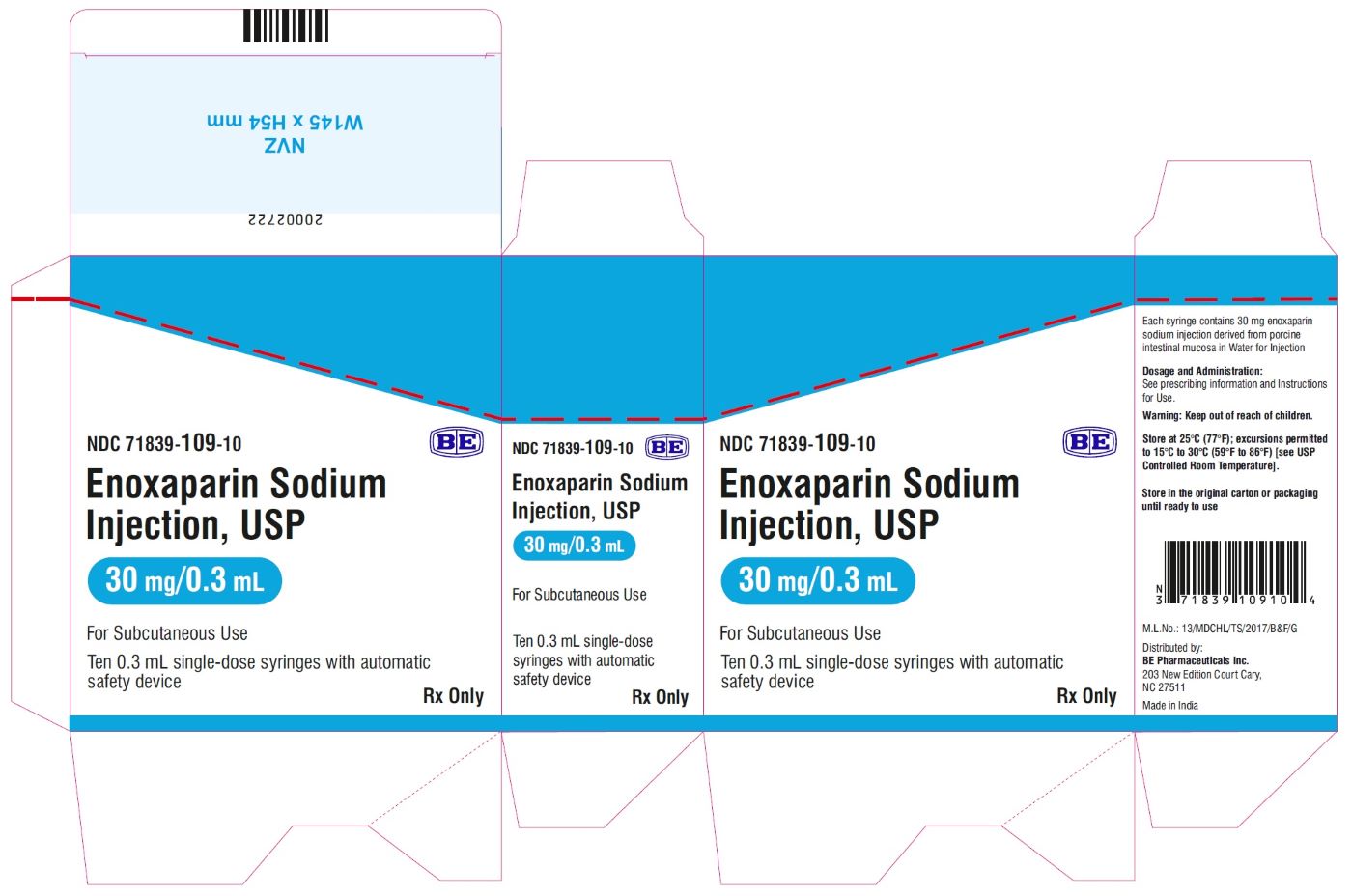
Enoxaparin Sodium Injection USP, 100 mg/mL (40 mg/0.4 mL) - Carton Label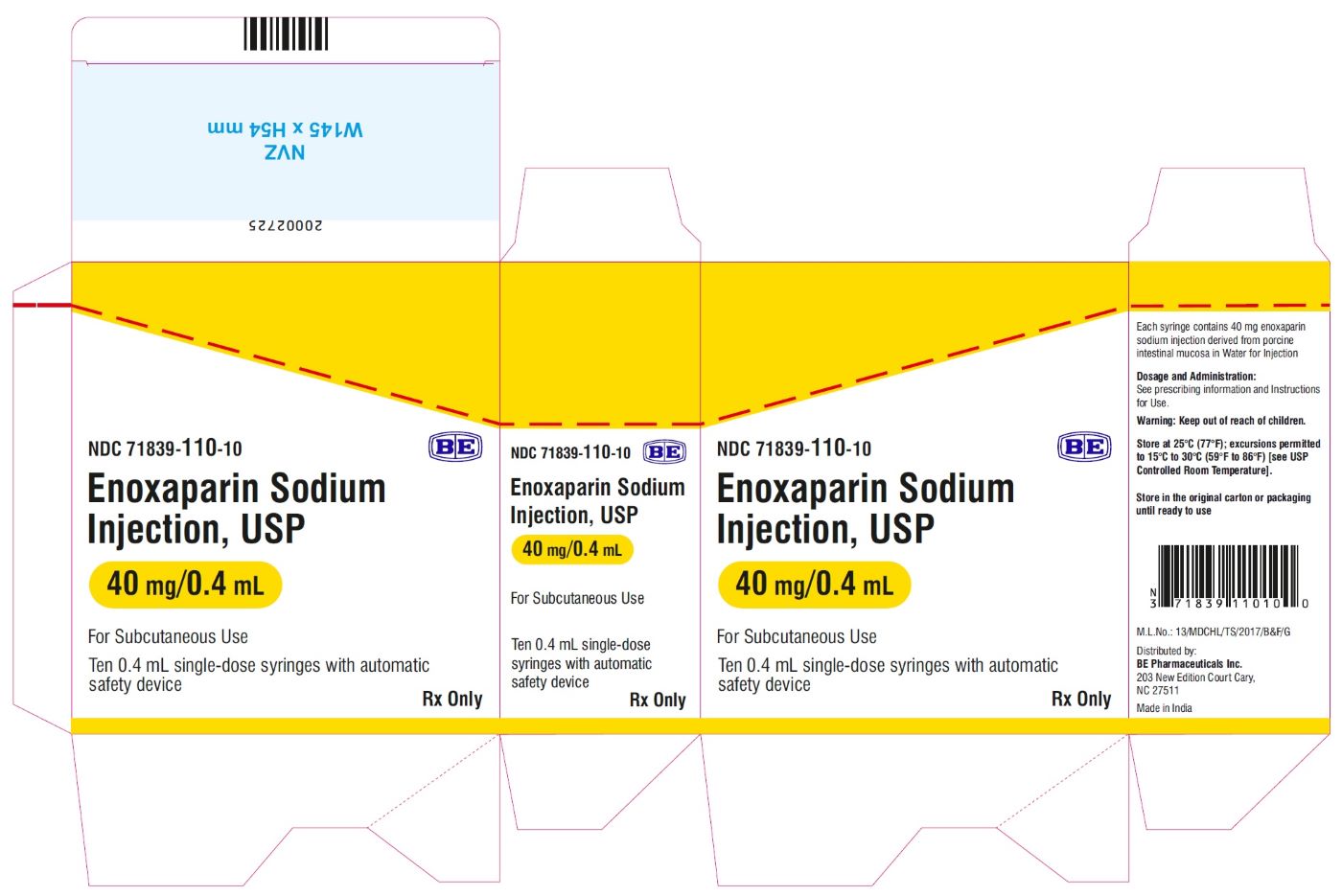
Enoxaparin Sodium Injection USP, 100 mg/mL (60 mg/0.6 mL) - Carton Label
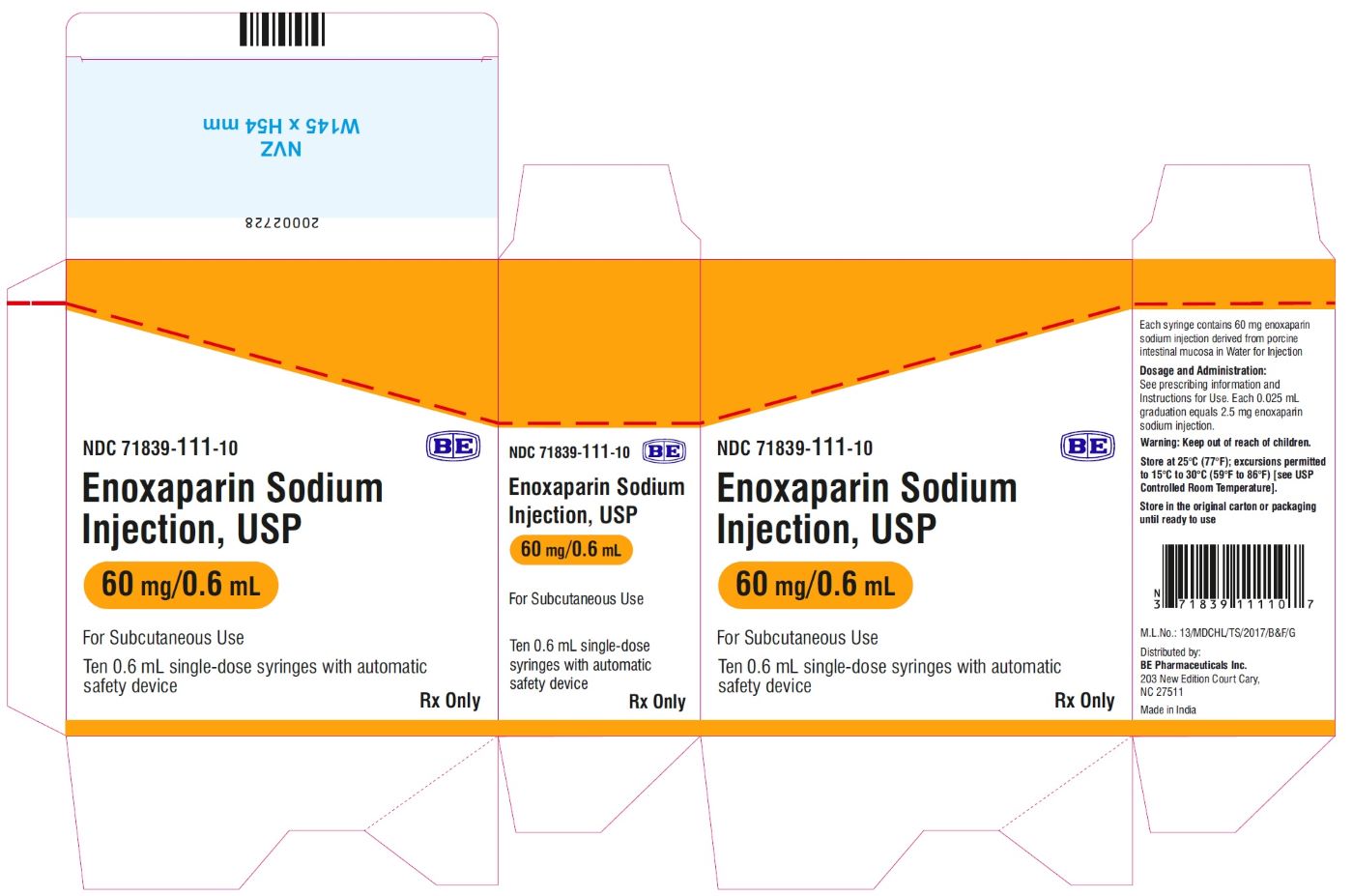
Enoxaparin Sodium Injection USP, 100 mg/mL (80 mg/0.8 mL) - Carton Label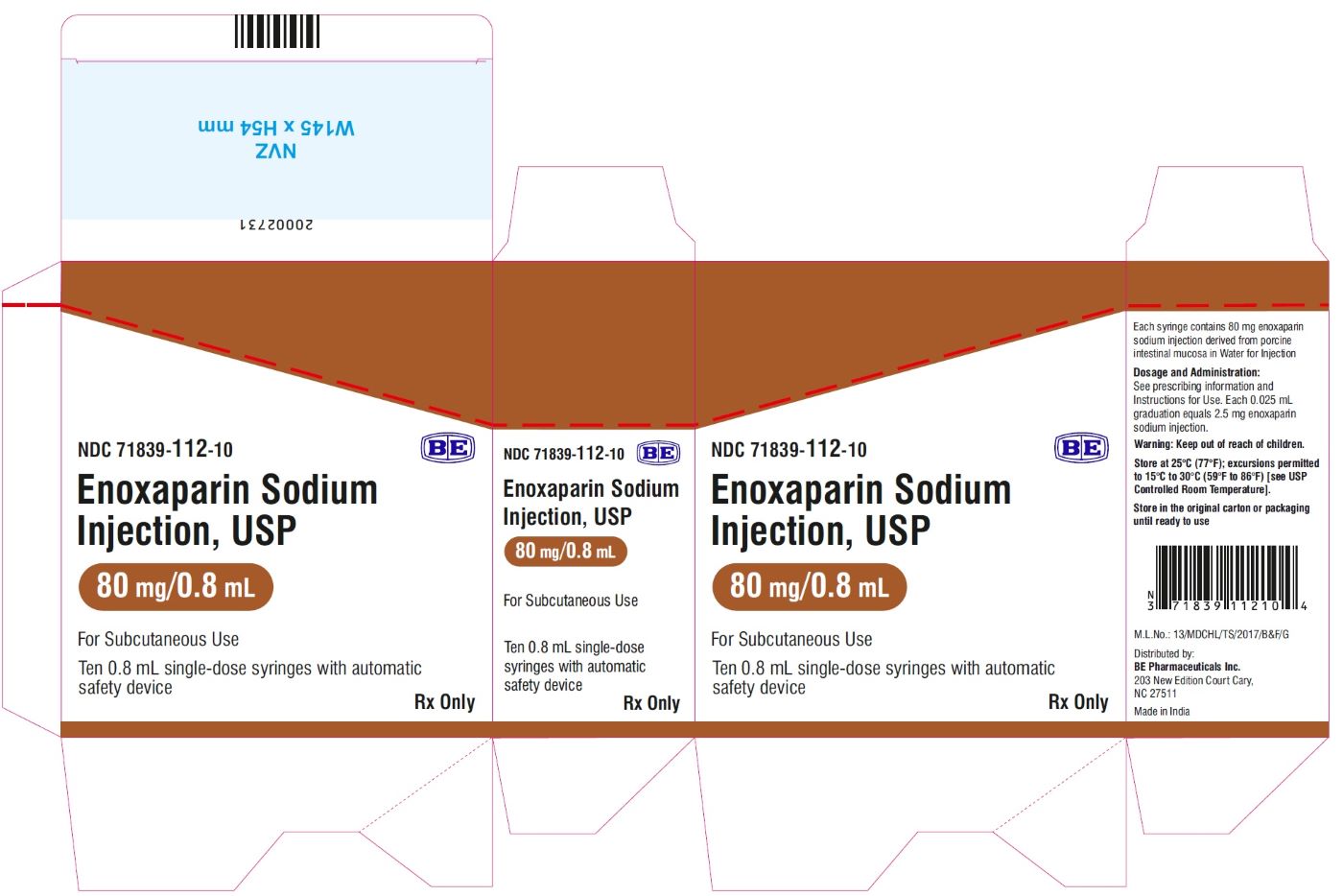
Enoxaparin Sodium Injection USP, 100 mg/mL (100 mg/1 mL) - Carton Label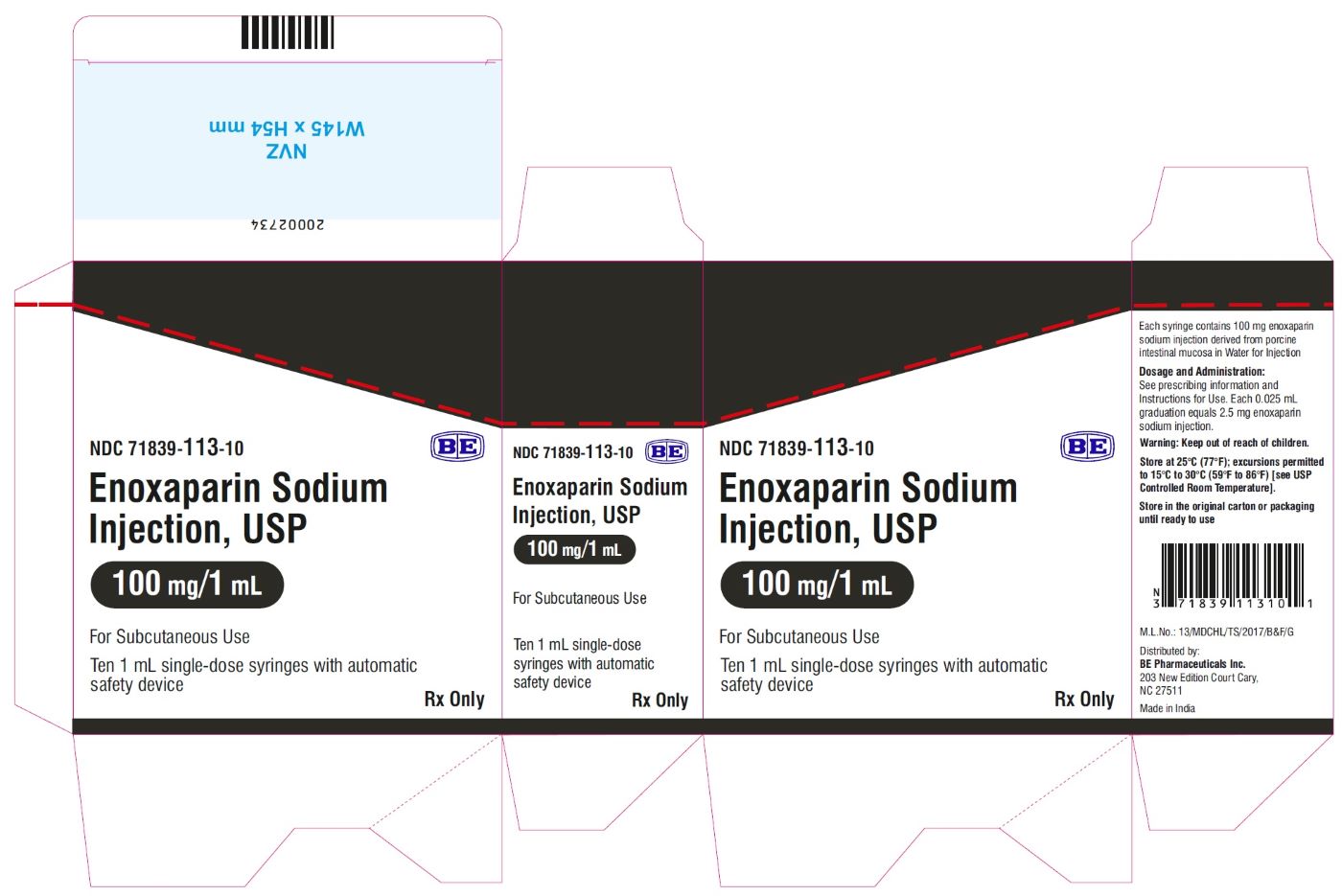
Enoxaparin Sodium Injection USP, 150 mg/mL (120 mg/0.8 mL) - Carton Label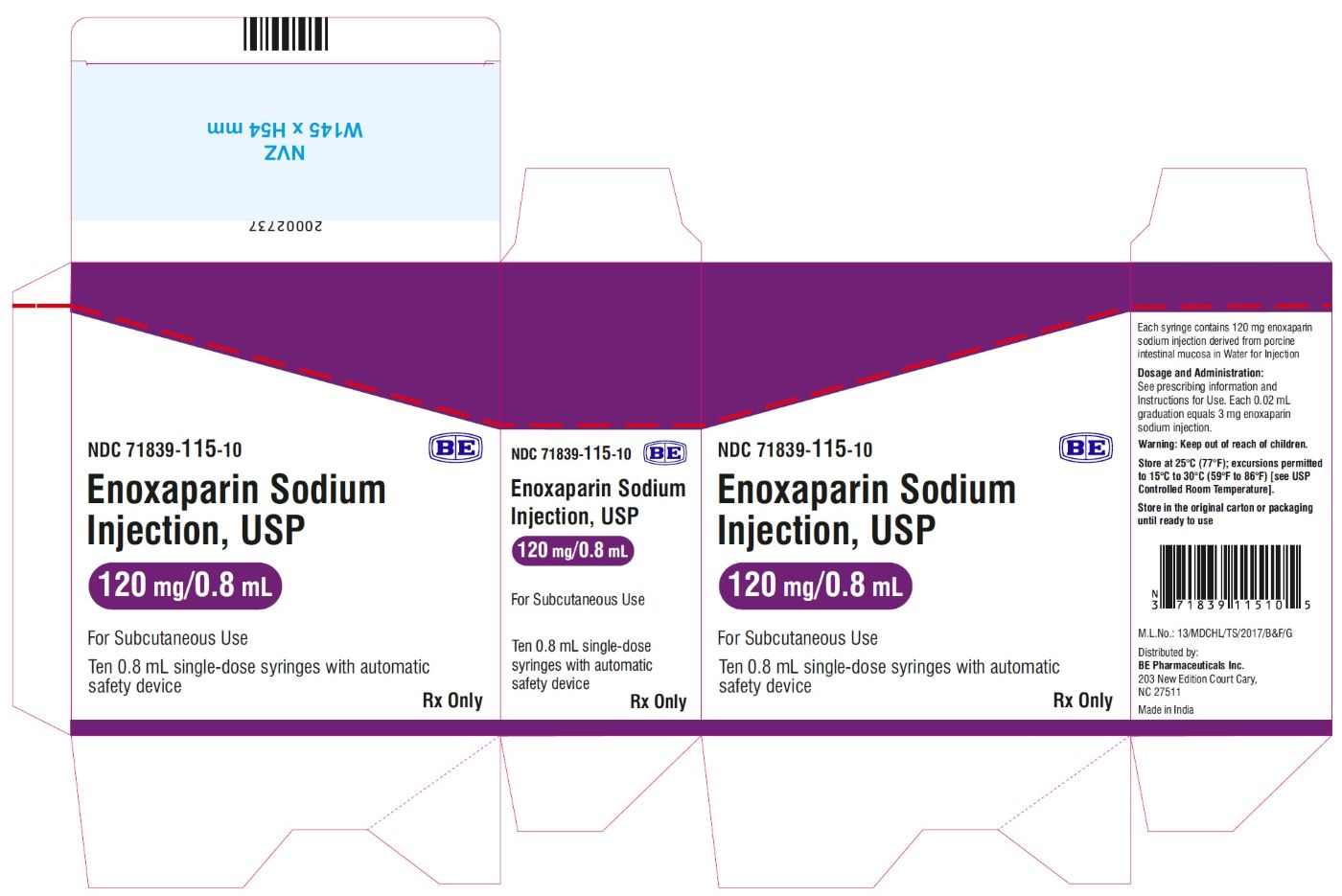
Enoxaparin Sodium Injection USP, 150 mg/mL (150 mg/1 mL) - Carton Label