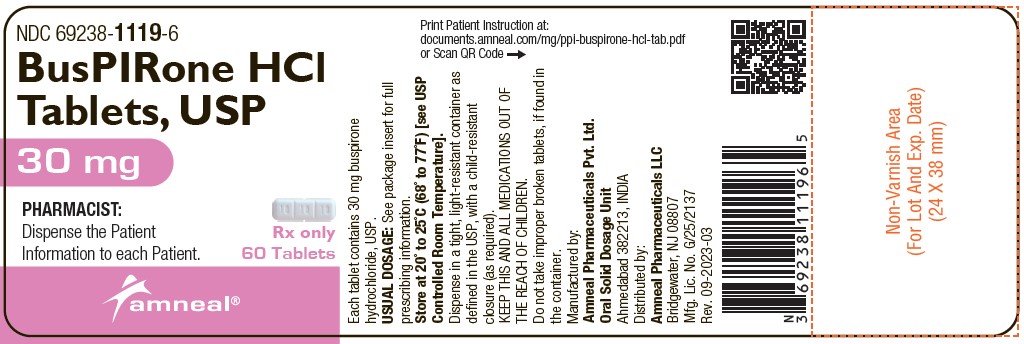
NDC Code(s) : 69238-1115-1, 69238-1115-5, 69238-1116-1, 69238-1116-5, 69238-1117-1, 69238-1117-5, 69238-1118-6, 69238-1118-1, 69238-1118-2, 69238-1118-5, 69238-1119-6, 69238-1119-5
Packager : Amneal Pharmaceuticals NY LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| busPIRone HClbuspirone hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| busPIRone HClbuspirone hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| busPIRone HClbuspirone hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| busPIRone HClbuspirone hydrochloride TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| busPIRone HClbuspirone hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Amneal Pharmaceuticals NY LLC(123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Amneal Pharmaceuticals Private Limited | 650762060 | manufacture(69238-1115, 69238-1116, 69238-1117, 69238-1118, 69238-1119), pack(69238-1115, 69238-1116, 69238-1117, 69238-1118, 69238-1119) | |
PRINCIPAL DISPLAY PANEL