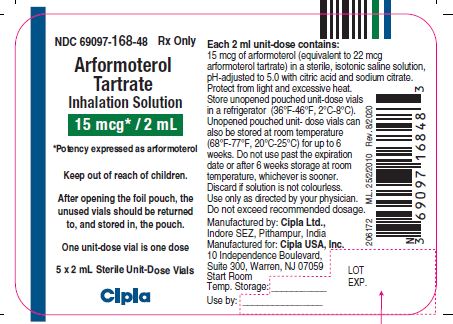
NDC Code(s) : 69097-168-48, 69097-168-87, 69097-168-64, 69097-168-32, 69097-168-53
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| arformoterol tartrate arformoterol tartrate SOLUTION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Limited- Kurkumbh | 917066446 | API MANUFACTURE(69097-168) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Ltd. Indore | 918596409 | ANALYSIS(69097-168), MANUFACTURE(69097-168), LABEL(69097-168), PACK(69097-168) | |
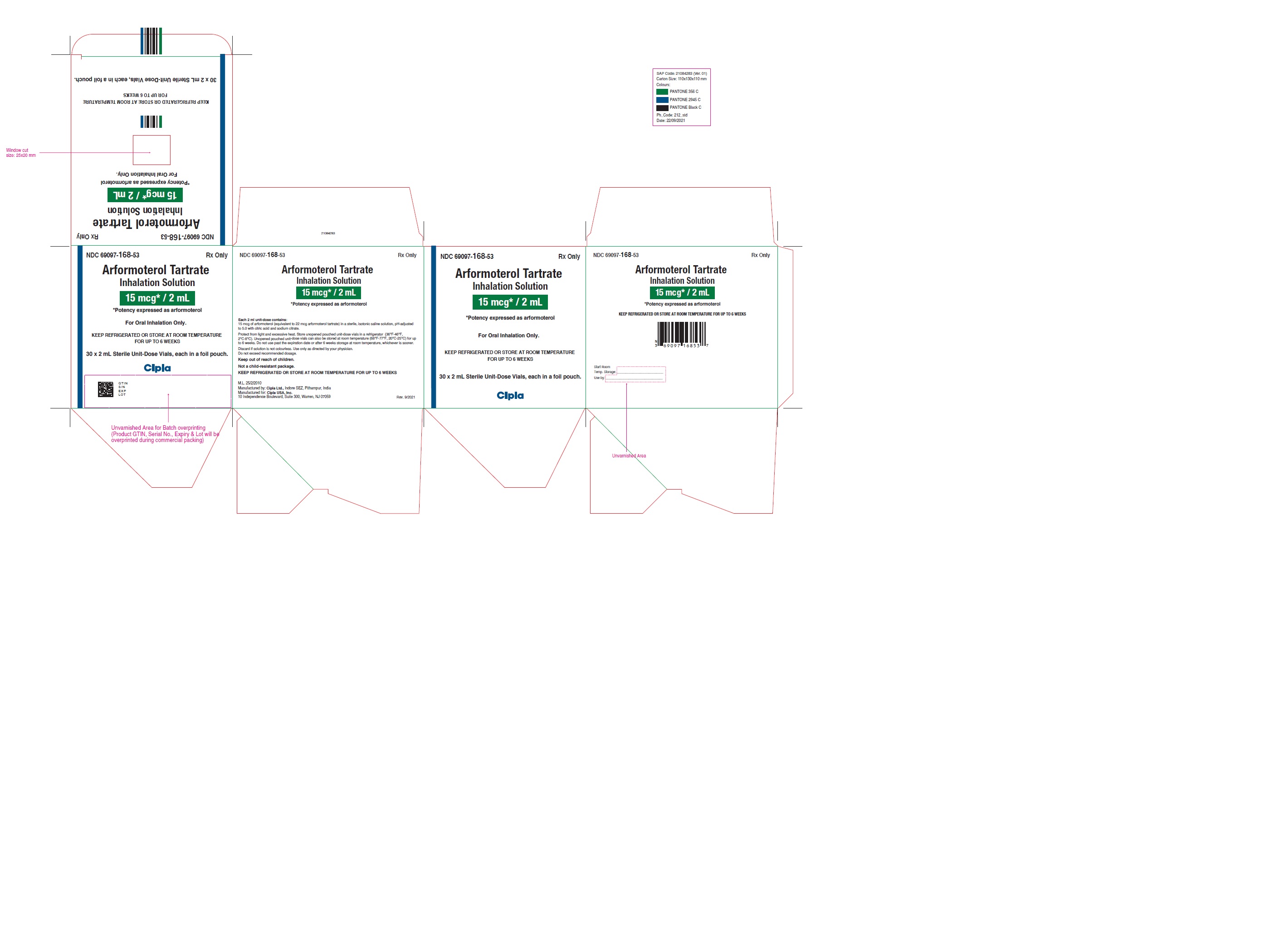
PRINCIPAL DISPLAY PANEL
NDC 69097-168-48 Rx Only
Arformoterol
Tartrate
Inhalation Solution
15mcg/2ml
*Potency expressed as arformoterol
Keep out of reach of children.
After opening the foil pouch, the
unused vials should be returned
to, and stored in, the pouch.
One unit-dose vial is one dose
5 X 2 mL Sterile Unit-Dose Vials
Cipla
NDC 69097-168-64 Rx Only
Arformoterol Tartrate
Inhalation Solution
15mcg*/2mL
*Potency expressed as arformoterol
For Oral Inhalation Only.
KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE FOR UP TO 6 WEEKS
6 0 (12 x 5) x 2 mL Sterile Unit- Dose Vials
Cipla

NDC 69097-168-87 Rx Only
Arformoterol Tartrate
Inhalation Solution
15mcg*/2mL
*Potency expressed as arformoterol
For Oral Inhalation Only.
KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE FOR UP TO 6 WEEKS
30 (6 x 5) x 2 mL Sterile Unit- Dose Vials
Cipla

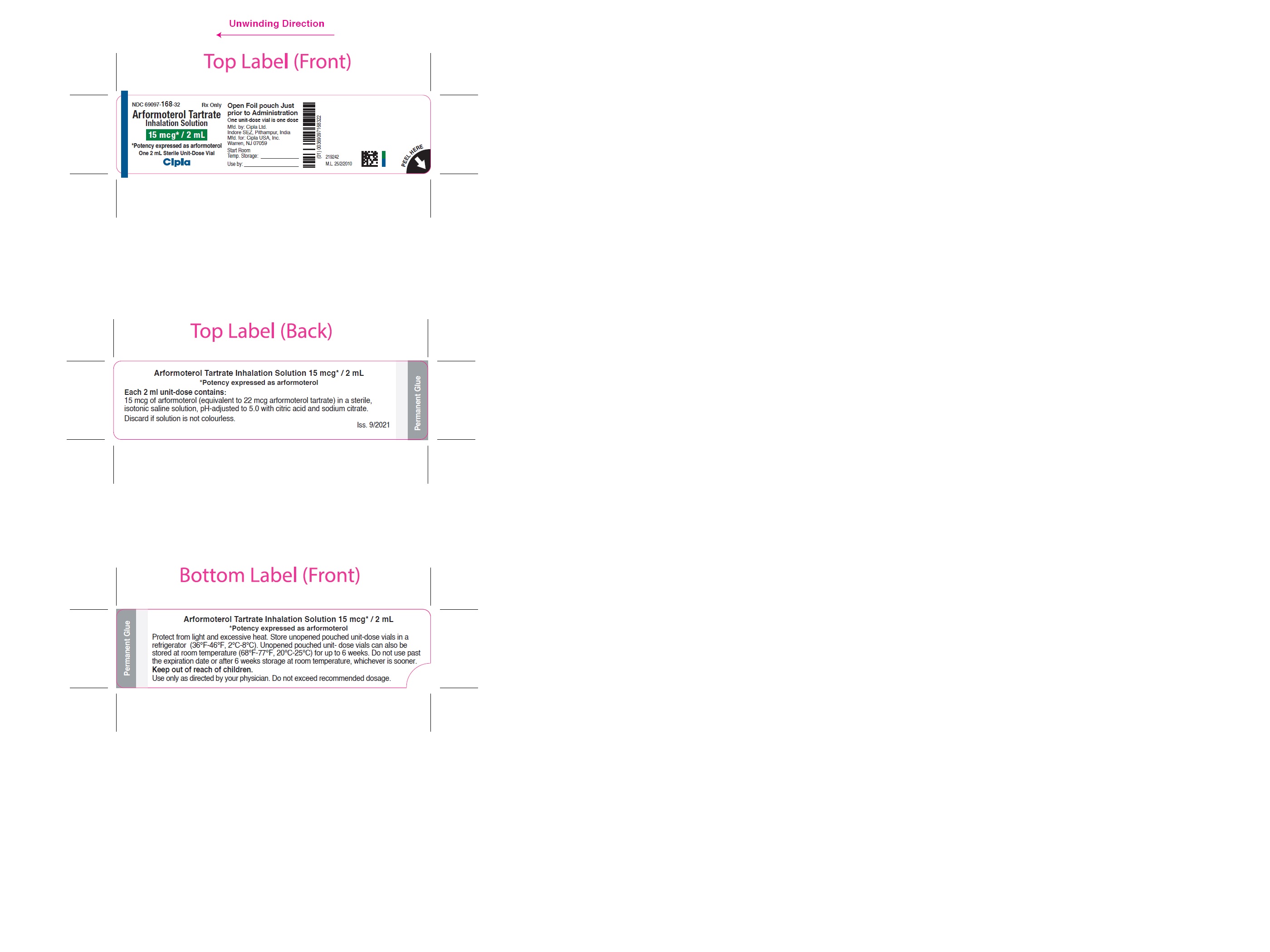
NDC 69097-168-32 Rx Only
Arformoterol Tartrate
Inhalation Solution
15 mcg* / 2 mL
Potency expressed as arformoterol
One 2 mL Sterile Unit-Dose Vial
Cipla
NDC 69097-168-53 Rx Only
*Potency expressed as arformoterol
15 mcg* / 2 mL
For Oral Inhalation Only.
KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE
FOR UP TO 6 WEEKS
30 x 2 mL Sterile Unit-Dose Vials, each in a foil pouch.
Cipla