NDC Code(s) : 68546-317-00, 68546-317-30, 68546-325-06, 68546-325-12
Packager : Teva Neuroscience, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CopaxoneGlatiramer Acetate INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| CopaxoneGlatiramer Acetate INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Teva Neuroscience, Inc.(009906397) |
PRINCIPAL DISPLAY PANEL
NDC 68546-317-30
ONCE DAILY
COPAXONE®
(glatiramer acetate injection) 20 mg/mL
Rx Only
30 Single-Dose Pre-Filled Syringes
Each pre-filled syringe contains 1 mL COPAXONE® solution of: glatiramer acetate 20 mg; mannitol 40 mg (inactive ingredient)
FOR SUBCUTANEOUS INJECTION ONLY
Marketed by: Teva Neuroscience, Inc., Parsippany, NJ 07054
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany NJ, 07054
Product of Israel

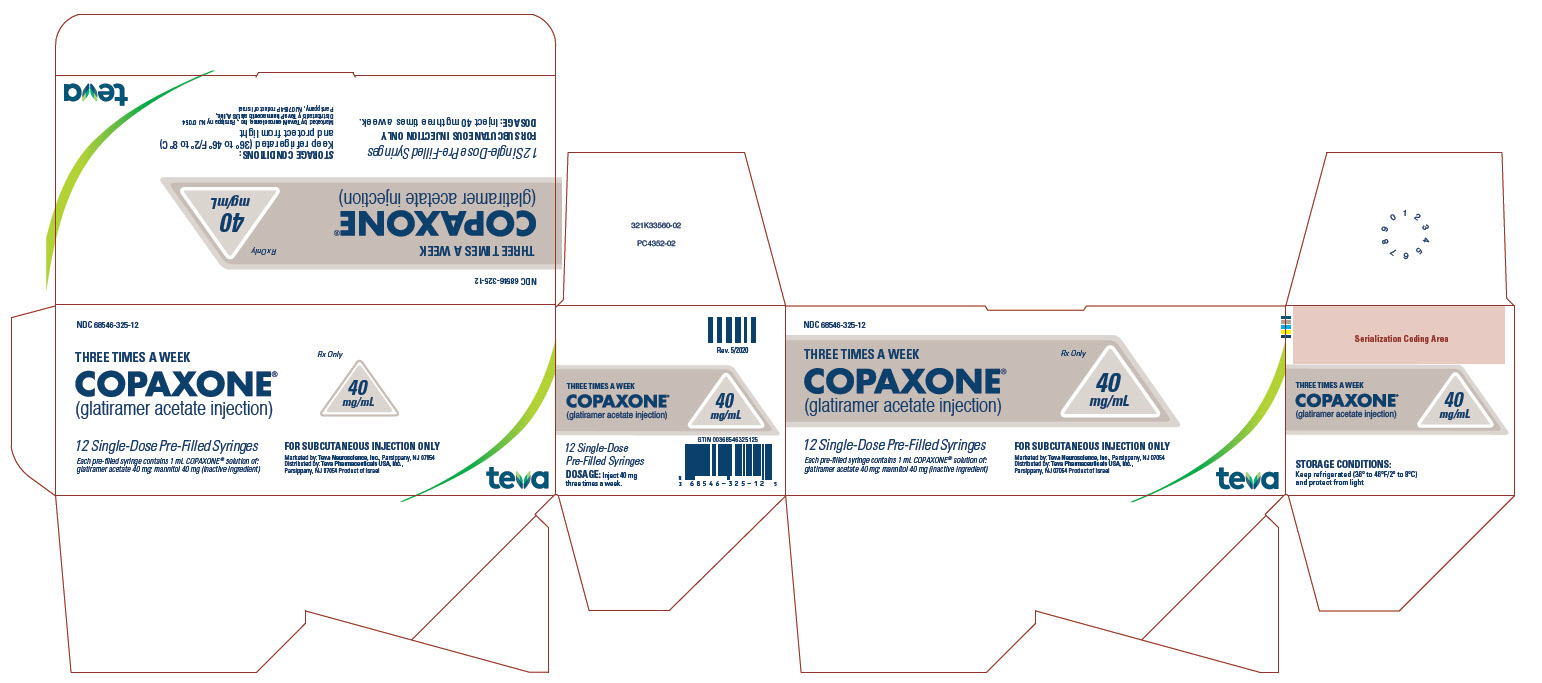
PRINCIPAL DISPLAY PANEL
NDC 68546-325-12
THREE TIMES A WEEK
COPAXONE®
(glatiramer acetate injection)
40
mg/mL
Rx Only
12 Single-Dose Pre-Filled Syringes
Each pre-filled syringe contains 1 mL COPAXONE® solution of: glatiramer acetate 40 mg; mannitol 40 mg (inactive ingredient)
FOR SUBCUTANEOUS INJECTION ONLY
Marketed by: Teva Neuroscience, Inc., Parsippany, NJ 07054
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054
Product of Israel