NDC Code(s) : 67877-743-95, 67877-744-95, 67877-744-54, 67877-745-54
Packager : Ascend Laboratories, LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| varenicline varenicline tartrate TABLET, FILM COATED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| varenicline varenicline tartrate TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| VareniclineVarenicline tartrate KIT | ||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Ascend Laboratories, LLC(141250469) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alkem Laboratories Limited | 677605851 | ANALYSIS(67877-743, 67877-744, 67877-745), MANUFACTURE(67877-743, 67877-744, 67877-745), PACK(67877-743, 67877-744, 67877-745) | |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL- STARTING MONTH BOX- BLISTER CARD
NDC 67877-745-54
Rx Only
Varenicline Tablets
STARTING MONTH BOX (Your first 4 weeks)

PRINCIPAL DISPLAY PANEL- STARTING MONTH BOX- CARTON
NDC 67877-745-54
Rx Only
Varenicline Tablets
STARTING MONTH BOX (Your first 4 weeks)

PRINCIPAL DISPLAY PANEL- CONTINUING MONTH BOX- BLISTER CARD
NDC 67877-744-54
Rx Only
Varenicline Tablets
CONTINUING PACK
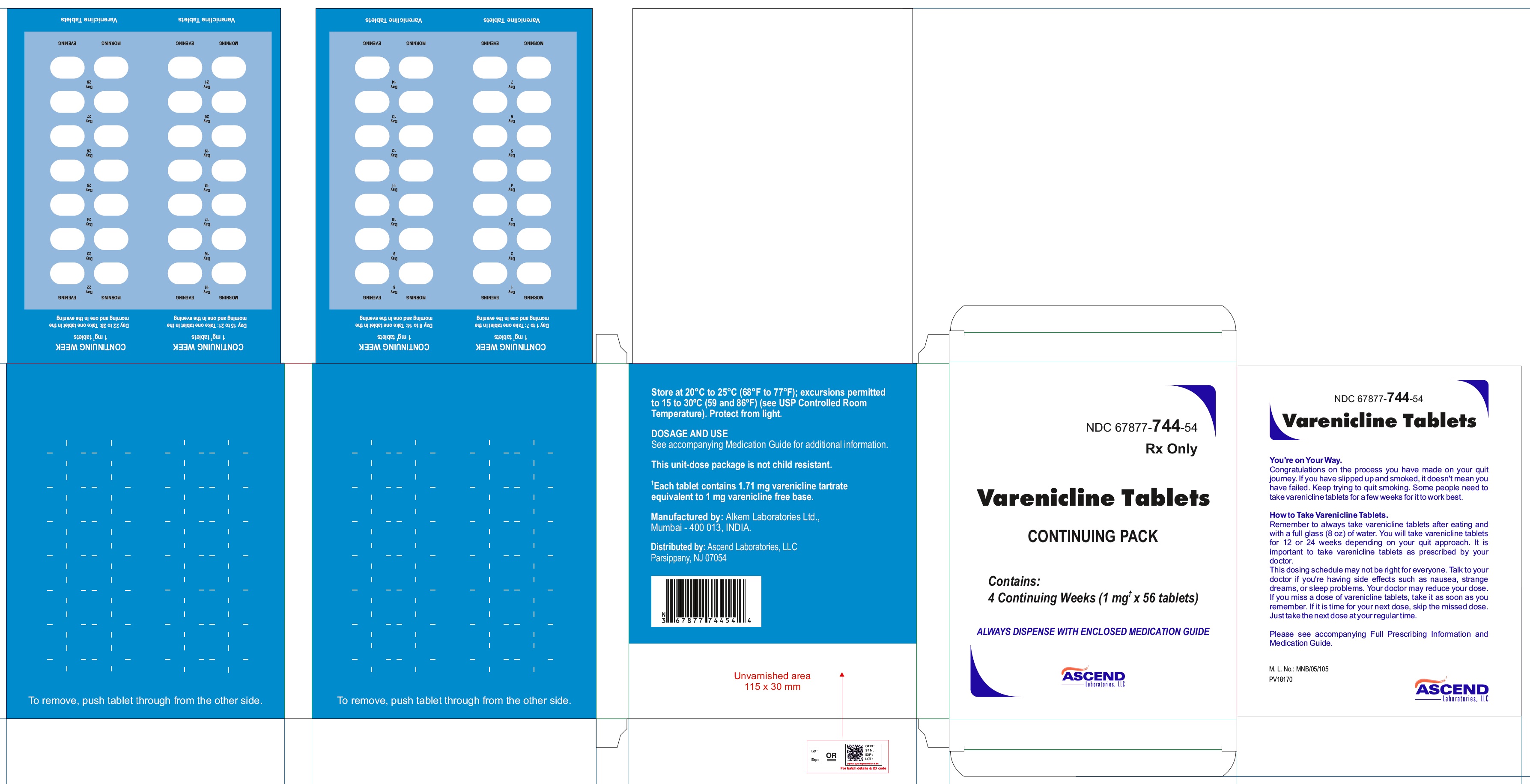
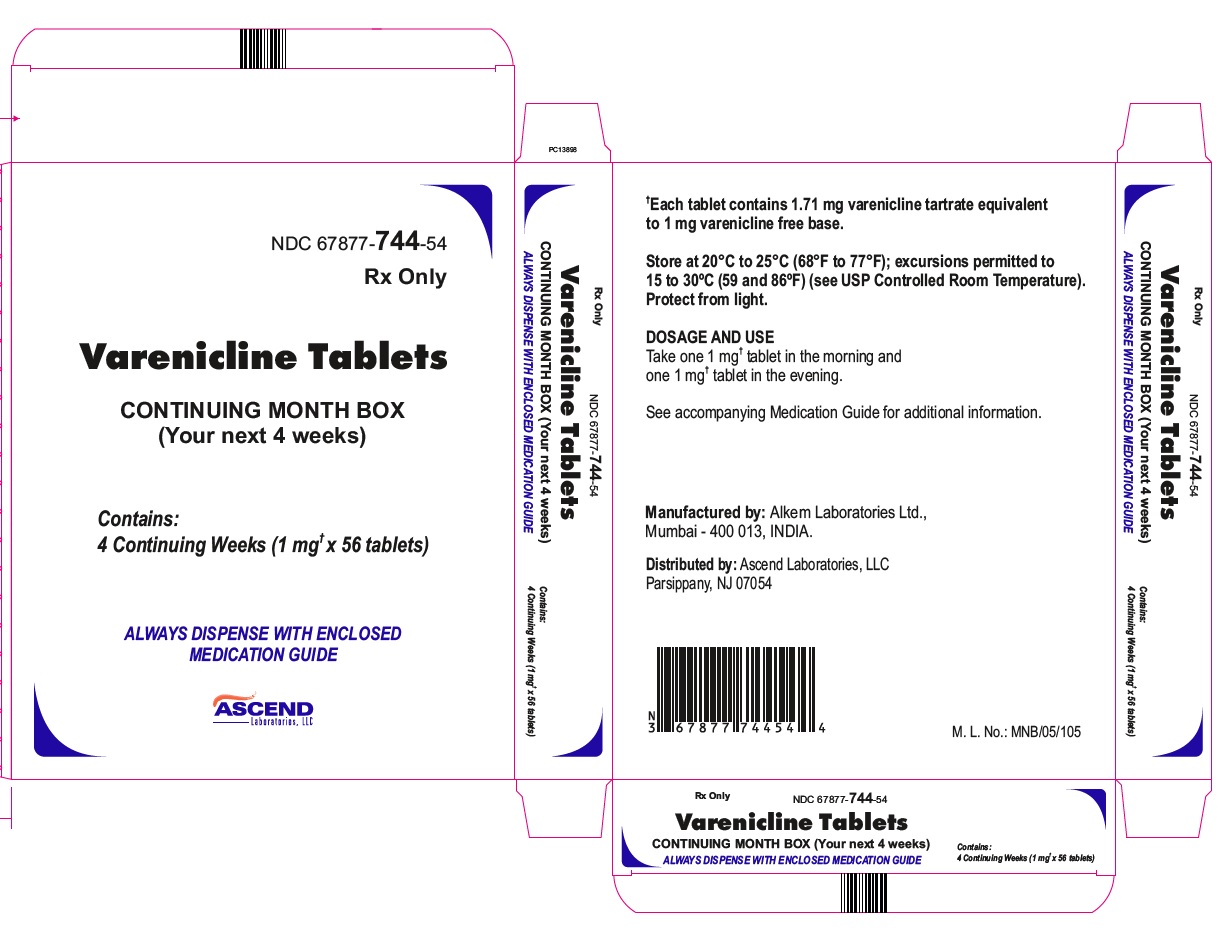
PRINCIPAL DISPLAY PANEL- CONTINUING MONTH BOX- CARTON
NDC 67877-744-54
Rx Only
Varenicline Tablets
CONTINUING MONTH BOX (Your next 4 weeks)
PRINCIPAL DISPLAY PANEL- 0.5 MG BOTTLE PACK OF 56 TABLETS
NDC 67877-743-95
Varenicline Tablets

PRINCIPAL DISPLAY PANEL- 1 MG BOTTLE PACK OF 56 TABLETS
NDC 67877-744-95
Varenicline Tablets










