NDC Code(s) : 67877-455-30, 67877-455-90, 67877-455-05, 67877-455-34, 67877-455-94
Packager : Ascend Laboratories, LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Finasteride Finasteride TABLET, COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Ascend Laboratories, LLC(141250469) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alkem Laboratories Limited | 677605851 | ANALYSIS(67877-455), MANUFACTURE(67877-455), PACK(67877-455) | |
PRINCIPAL DISPLAY PANEL
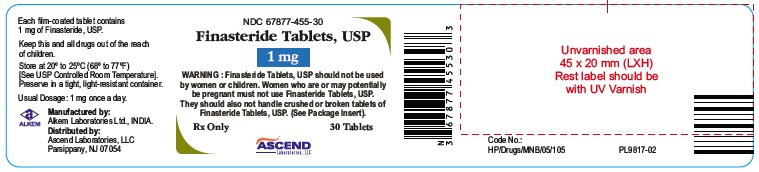
Finasteride Tablets, USP 1 mg
Warning: Finasteride Tablets, USP 1 mg should not be used by women or children. Women who are or may potentially be pregnant must not use Finasteride Tablets, USP 1 mg. They should also not handle crushed or broken tablets of Finasteride Tablets, USP 1 mg. (See Package Insert.)
Rx only
30 Tablets
Finasteride Tablets, USP 1 mg
Warning: Finasteride Tablets, USP 1 mg should not be used by women or children. Women who are or may potentially be pregnant must not use Finasteride Tablets, USP 1 mg. They should also not handle crushed or broken tablets of Finasteride Tablets, USP 1 mg. (See Package Insert.)
Rx only
90 Tablets

Finasteride Tablets, USP 1 mg
Warning: Finasteride Tablets, USP 1 mg should not be used by women or children. Women who are or may potentially be pregnant must not use Finasteride Tablets, USP 1 mg. They should also not handle crushed or broken tablets of Finasteride Tablets, USP 1 mg. (See Package Insert.)
Rx only
500 Tablets










