NDC Code(s) : 62856-796-01, 62856-796-04
Packager : Eisai Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AKYNZEONetupitant and Palonosetron CAPSULE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
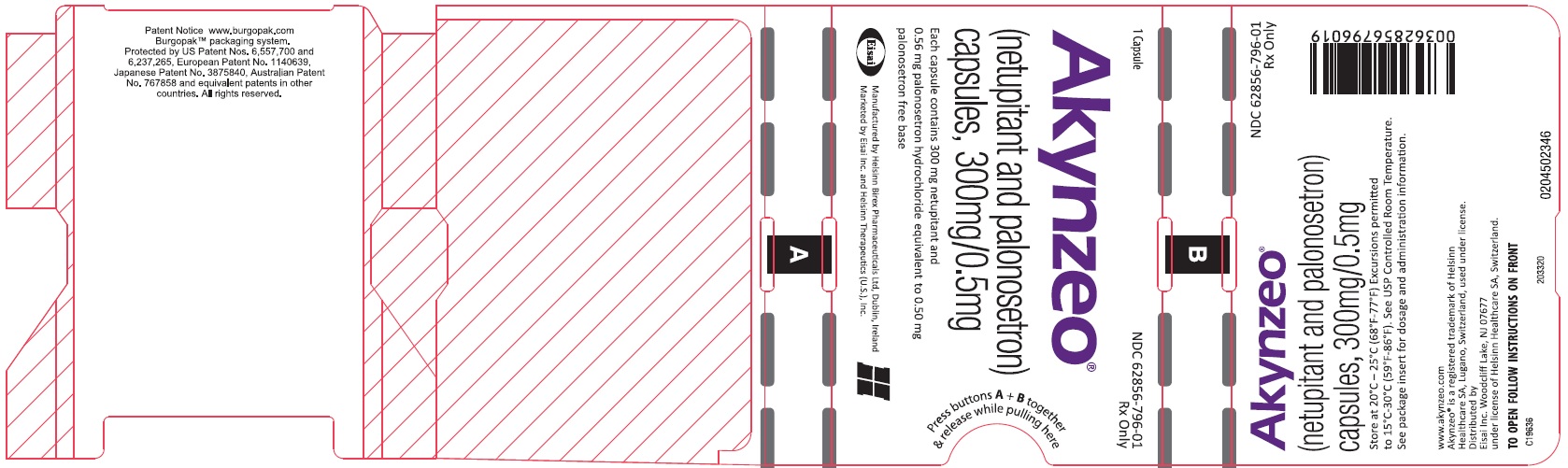
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 62856-796-01
Akynzeo
(netupitant and palonosetron)
capsules, 300mg/0.5mg

PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 62856-796-01
Akynzeo
(netupitant and palonosetron)
capsules, 300mg/0.5mg
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 62856-796-04
Akynzeo
(netupitant and palonosetron)
capsules, 300mg/0.5mg
For Hospital Use Only

PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 62856-796-04
Akynzeo
(netupitant and palonosetron)
capsules, 300mg/0.5mg
For Hospital Use Only










