NDC Code(s) : 62756-130-02, 62756-130-01, 62756-131-02, 62756-131-01, 62756-240-64, 62756-240-83, 62756-356-64, 62756-356-83, 62756-356-66
Packager : Sun Pharmaceutical Industries, Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ondansetron hydrochloride ondansetron hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ondansetron hydrochloride ondansetron hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ondansetron ondansetron TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ondansetron ondansetron TABLET, ORALLY DISINTEGRATING | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries Limited | 725959238 | ANALYSIS(62756-130, 62756-131, 62756-240, 62756-356), MANUFACTURE(62756-130, 62756-131, 62756-240, 62756-356) | |
PRINCIPAL DISPLAY PANEL
NDC 62756-130-01
Ondansetron Hydrochloride Tablets
4 mg*
Rx only
30 Tablets
SUN PHARMA
NDC 62756-131-01
Ondansetron Hydrochloride Tablets
8 mg*
Rx only
30 Tablets
SUN PHARMA
NDC 62756-130-02
Ondansetron Hydrochloride Tablets
4 mg
Rx only
100 (10x10) Unit Dose Tablets
UNIT DOSE
SUN PHARMA

NDC 62756-131-02
Ondansetron Hydrochloride Tablets
8 mg
Rx only
100 (10x10) Unit Dose Tablets
UNIT DOSE
SUN PHARMA
PRINCIPAL DISPLAY PANEL
NDC 62756-240-83
Ondansetron Orally Disintegrating Tablets
4 mg
Rx only
30 Tablets
SUN PHARMA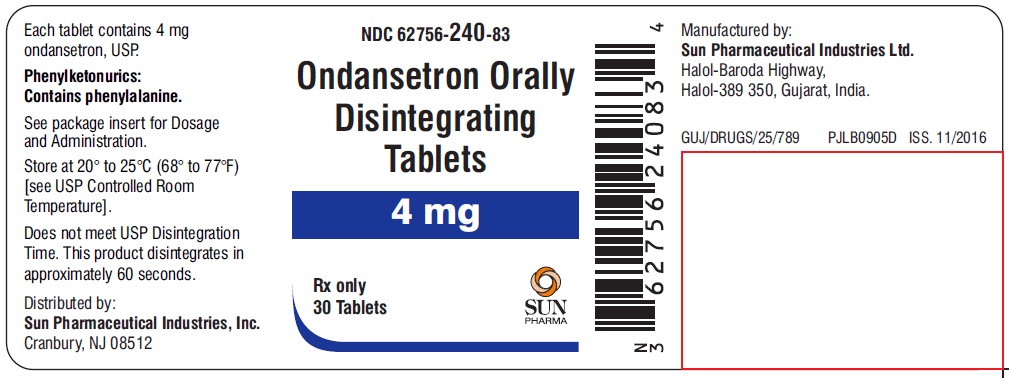
NDC 62756-356-83
Ondansetron Orally Disintegrating Tablets
8 mg
Rx only
30 Tablets
SUN PHARMA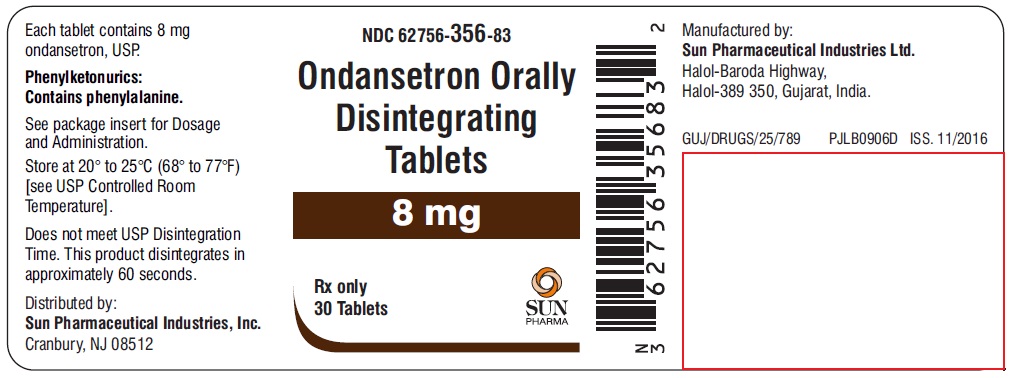
NDC 62756-240-64
Ondansetron Orally Disintegrating Tablets
4 mg
Rx only
30 Tablets
(3 blistercards each containing 10 tablets)
SUN PHARMA

NDC 62756-356-64
Ondansetron Orally Disintegrating Tablets
8 mg
Rx only
30 Tablets
(3 blistercards each containing 10 tablets)
SUN PHARMA










