NDC Code(s) : 62332-655-01, 62332-655-30, 62332-655-60
Packager : Alembic Pharmaceuticals Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Formoterol Fumarate Formoterol Fumarate SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals Inc.(079288842) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Holopack Verpackungstechnik GmbH | 343390324 | MANUFACTURE(62332-655) | |
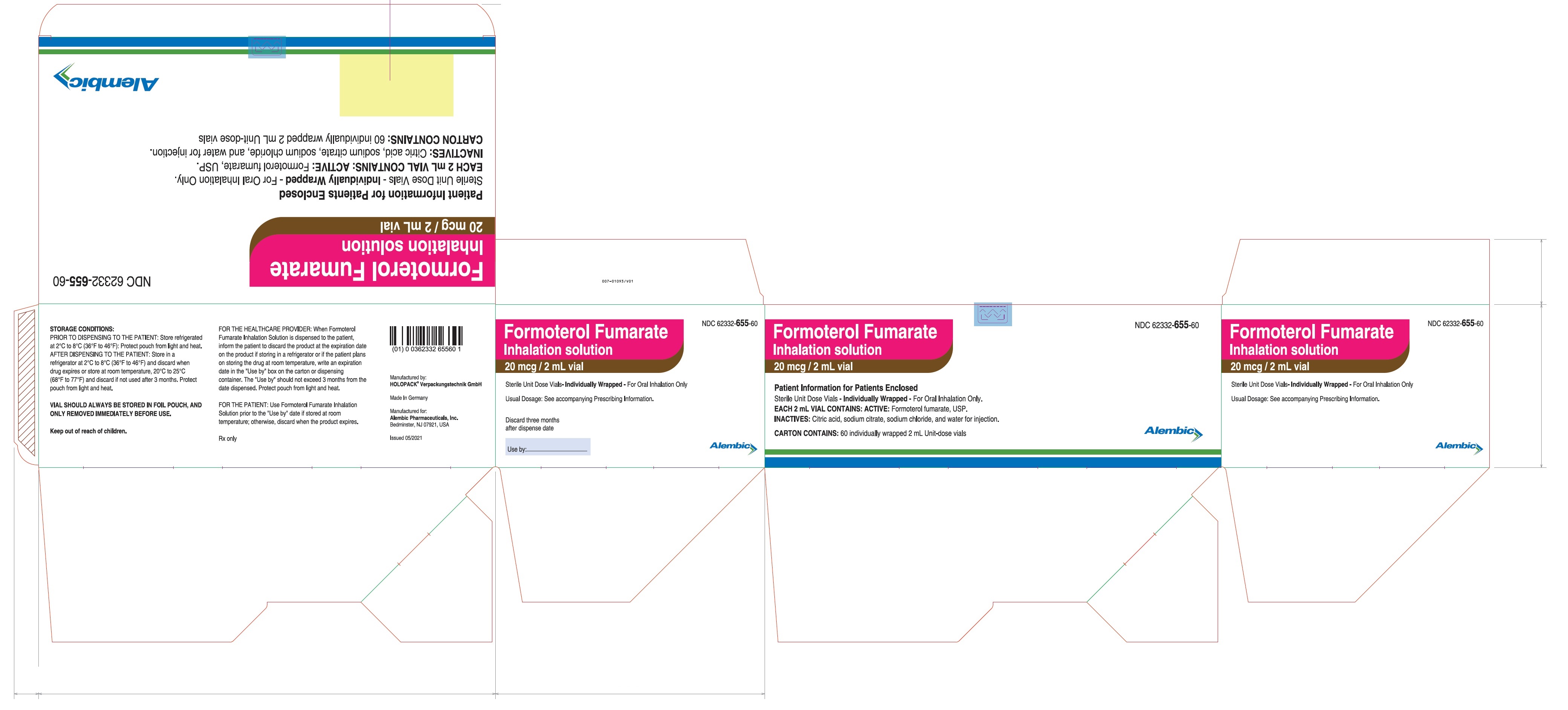
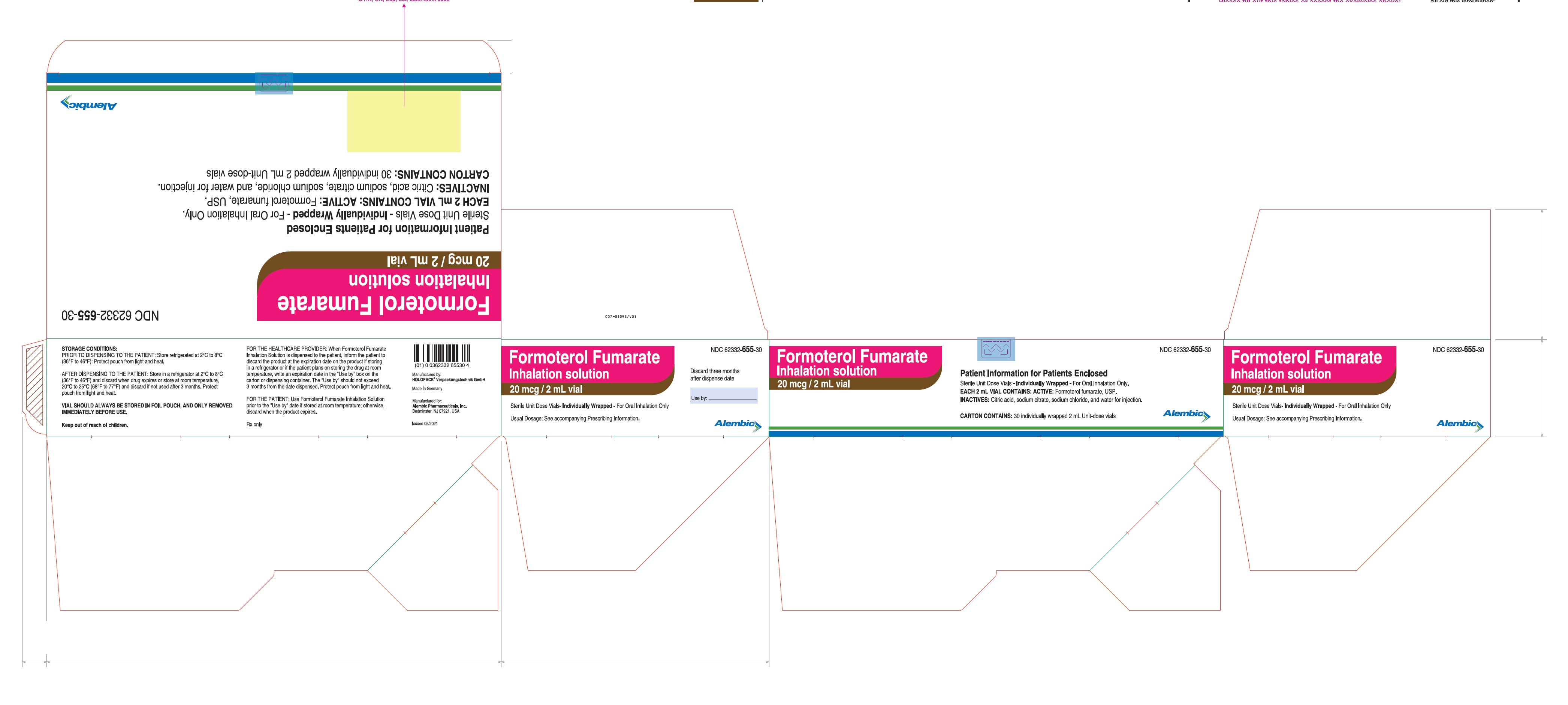
PRINCIPAL DISPLAY PANEL
Formoterol Fumarate Inhalation Solution - Pouch Label:

Formoterol Fumarate Inhalation Solution - Carton Label - 30's Pack:
Formoterol Fumarate Inhalation Solution - Carton Label - 60's Pack: