NDC Code(s) : 62332-630-45, 62332-630-60, 62332-630-55
Packager : Alembic Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Metronidazole Metronidazole GEL | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals, Inc.(079288842) |
| REGISTRANT - Alembic Pharmaceuticals Limited(650574663) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alembic Pharmaceuticals Limited | 871411532 | MANUFACTURE(62332-630), ANALYSIS(62332-630) | |
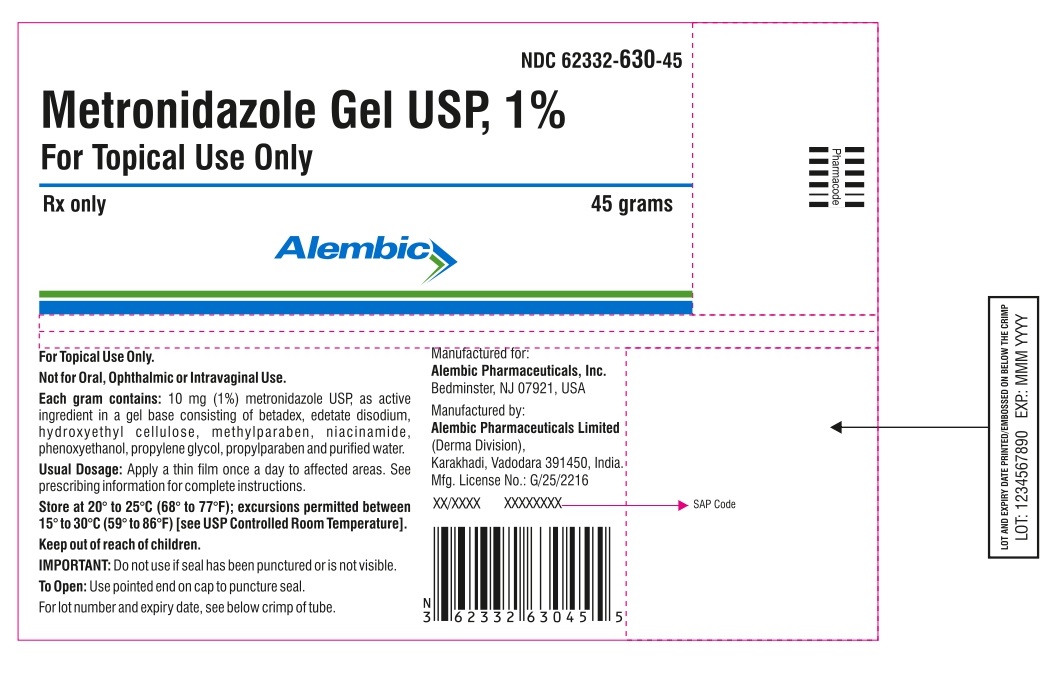
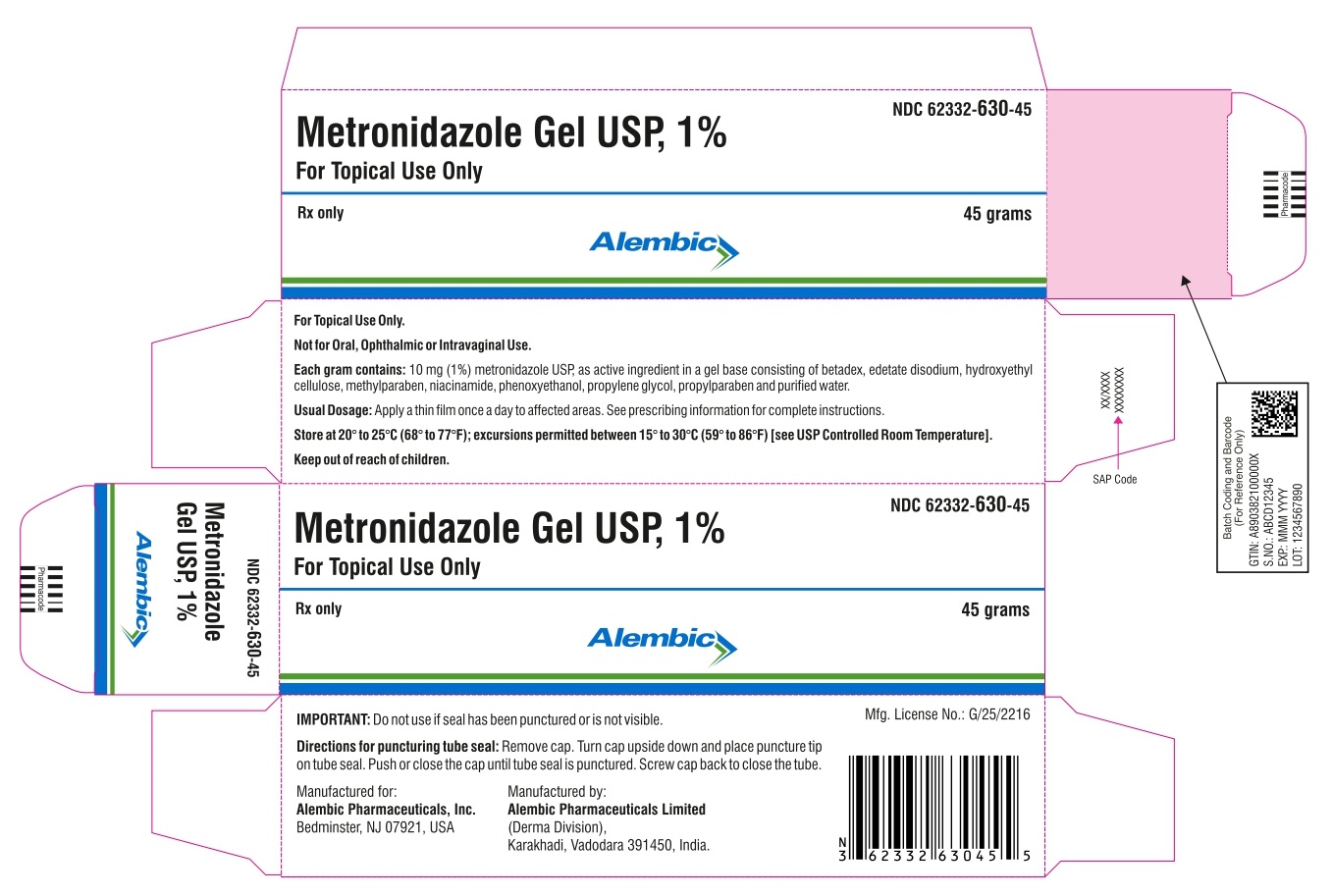
PRINCIPAL DISPLAY PANEL
Metronidazole Gel USP, 1%
For Topical Use Only
NDC 62332-630-45
Rx Only
45 grams
For topical use only.
Not for oral, ophthalmic or intravaginal use.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep out of reach of children.
Usual dosage: Apply a thin film once a day to affected areas. See prescribing information for complete instructions.
Each gram contains: 10 mg (1%) metronidazole USP, as active ingredient in a gel base consisting of betadex, edetate disodium, hydroxyethyl cellulose, methylparaben, niacinamide, phenoxyethanol, propylene glycol, propylparaben, and purified water.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Manufactured by:
Alembic Pharmaceuticals Limited
(Derma Division),
Karakhadi, Vadodara 391450, India.
Mfg. License No.: G/25/2216