NDC Code(s) : 60951-185-30, 60951-185-92, 60951-185-90, 60951-185-70, 60951-186-30, 60951-186-92, 60951-186-90, 60951-186-70, 60951-187-30, 60951-187-92, 60951-187-90, 60951-187-70
Packager : Par Pharmaceutical
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Losartan Potassium Losartan Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Losartan Potassium Losartan Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Losartan Potassium Losartan Potassium TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Losartan Potassium Tablets USP 25 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP...... 25 mg
60951-185-92

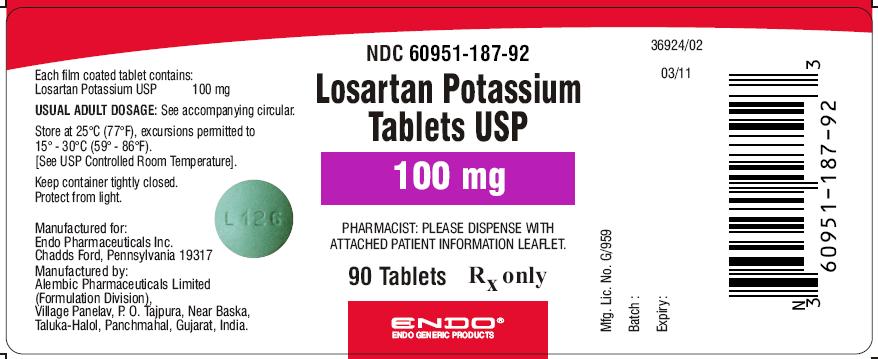
PRINCIPAL DISPLAY PANEL
Losartan Potassium Tablets USP 50 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP...... 50 mg
60951-186-92
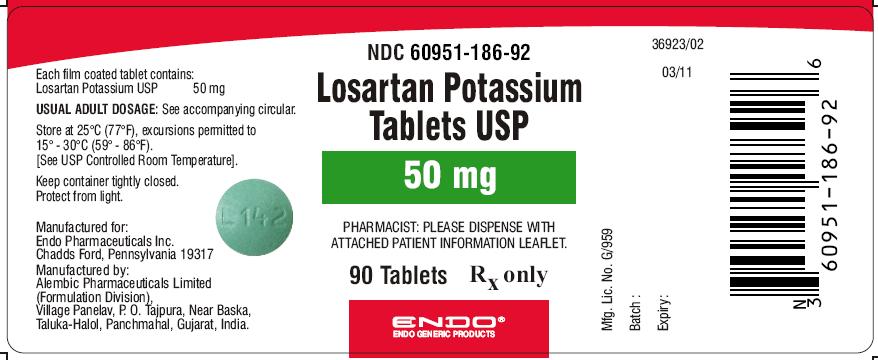
PRINCIPAL DISPLAY PANEL
Losartan Potassium Tablets USP 100 mg (90 Tablets in HDPE bottle)
Each film coated tablet contains: Losartan Potassium USP..... 100 mg
60951-187-92