NDC Code(s) : 60505-6144-4, 60505-6145-4
Packager : Apotex Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CefepimeCefepime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CefepimeCefepime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Apotex Corp.(845263701) |
| REGISTRANT - Qilu Pharmaceutical Co., Ltd.(653878256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Pharmaceutical Co., Ltd. (High-Tech Zone Site) | 421279342 | manufacture(60505-6144, 60505-6145), analysis(60505-6144, 60505-6145) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Antibiotics Pharmaceutical Co., Ltd. | 527271779 | manufacture(60505-6144, 60505-6145), analysis(60505-6144, 60505-6145) | |
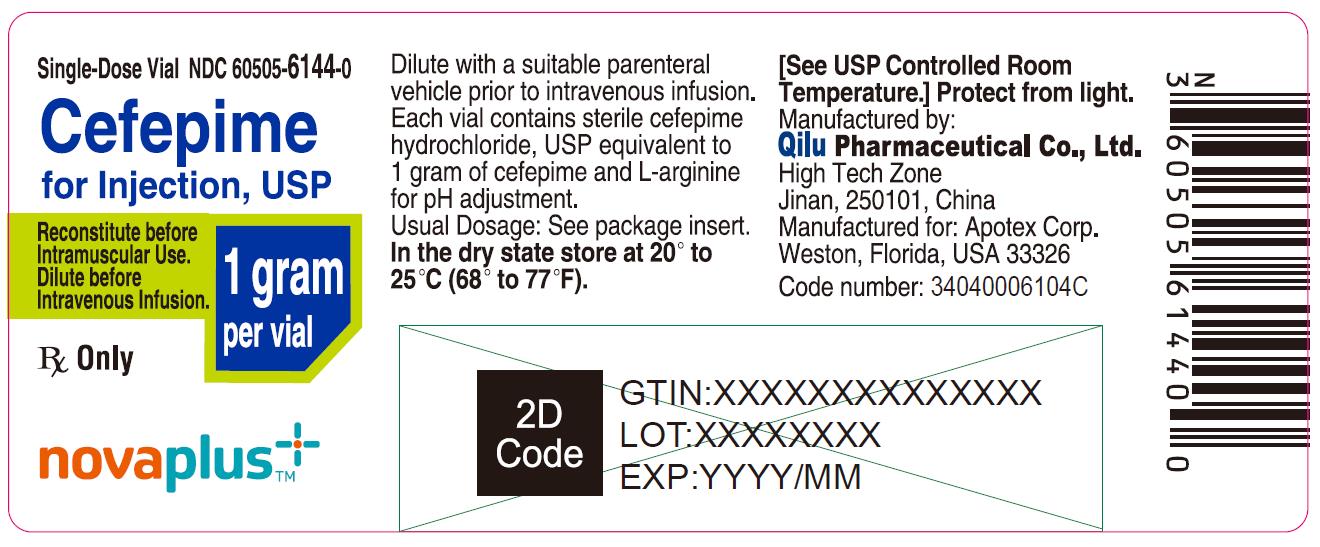
PRINCIPAL DISPLAY PANEL
Single-Dose Vial NDC 60505-6144-0
Cefepime for Injection, USP
1 gram per vial
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
Rx Only
NOVAPLUS®
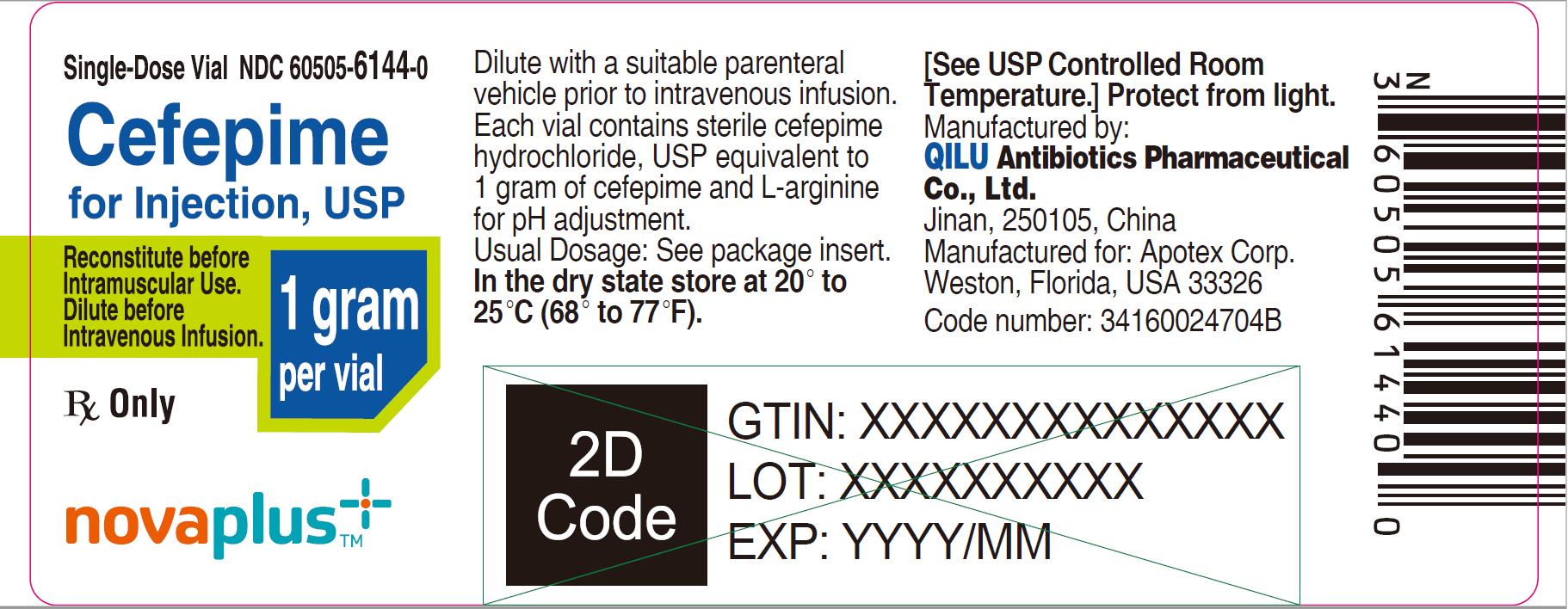
PRINCIPAL DISPLAY PANEL
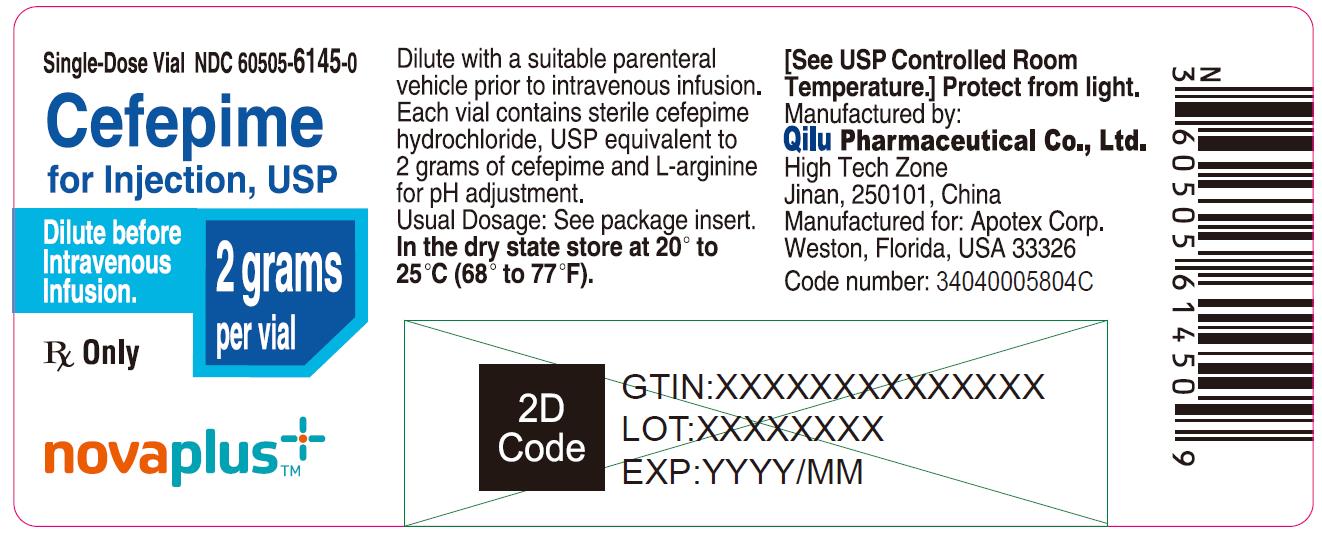
Single-Dose Vial NDC 60505-6145-0
Cefepime for Injection, USP
2 grams per vial
Dilute before Intravenous Infusion.
Rx Only
NOVAPLUS®

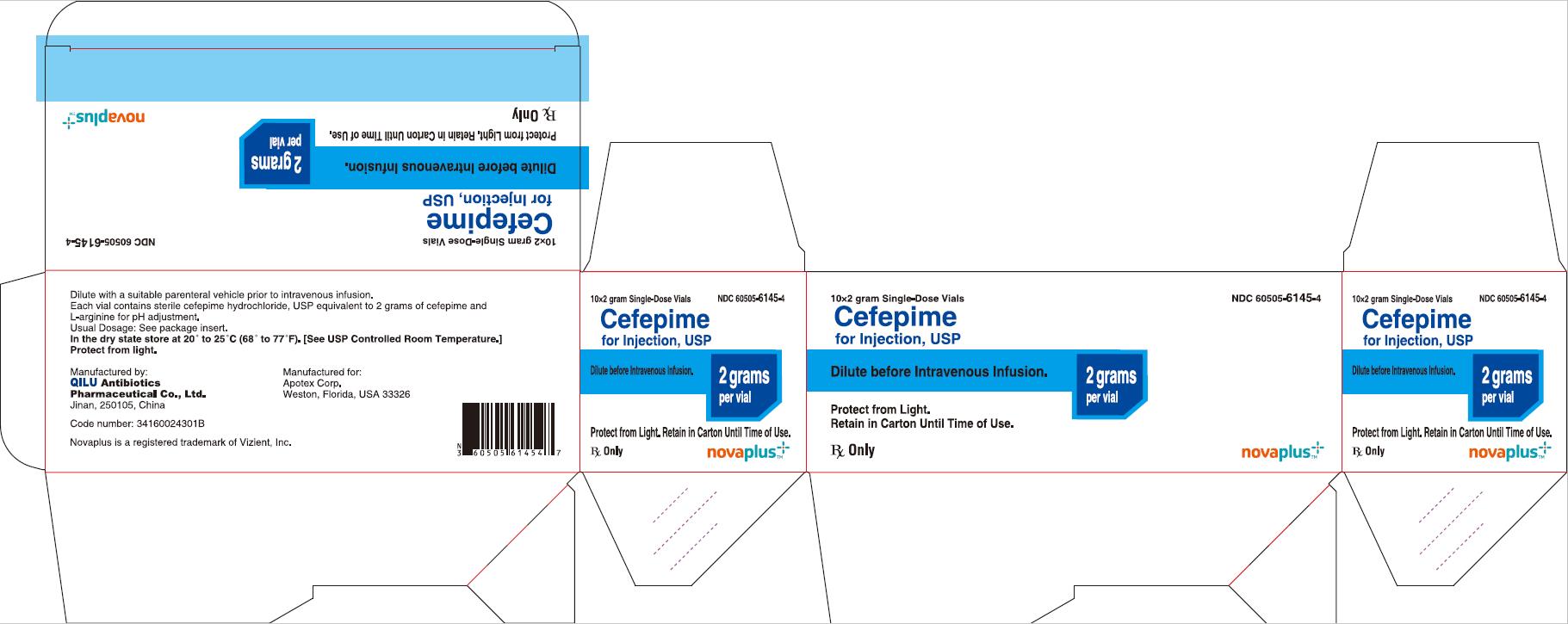
PRINCIPAL DISPLAY PANEL
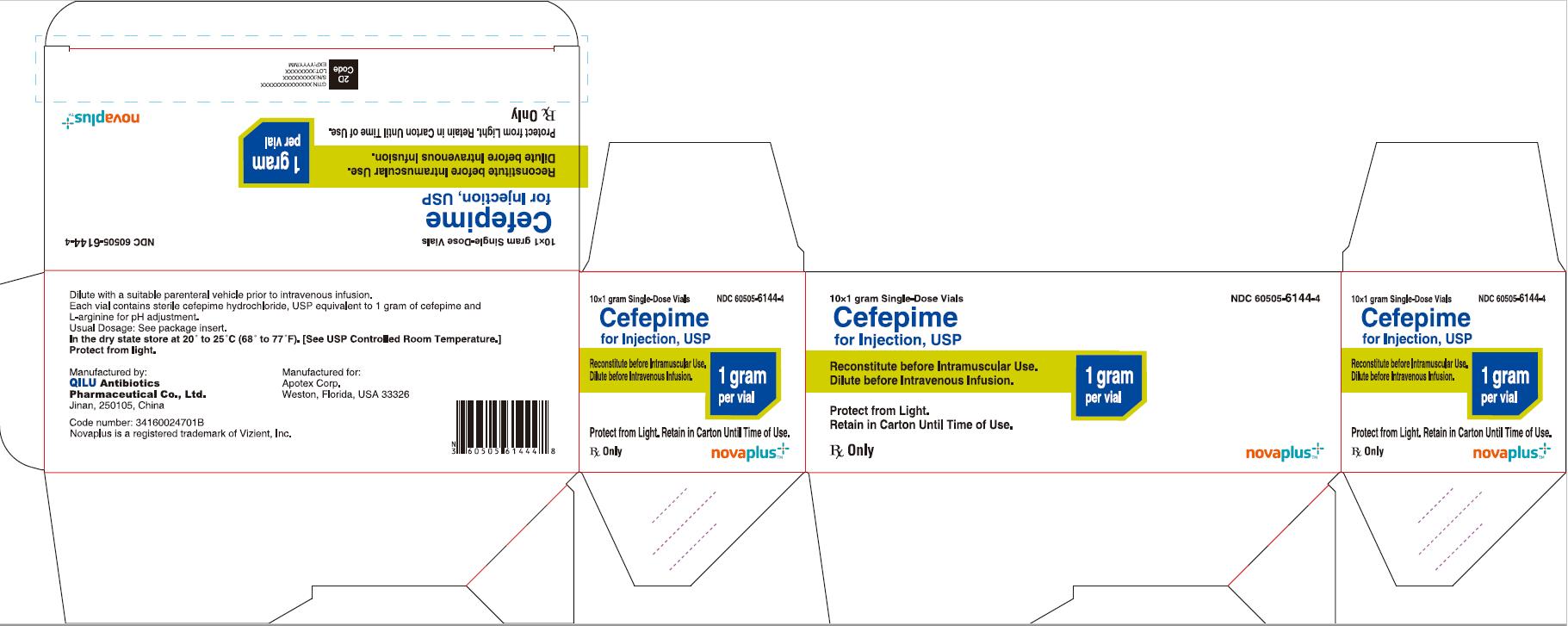
10*1 gram Single-Dose Vials NDC 60505-6144-4
Cefepime for Injection, USP
1 gram per vial
Reconstitute before Intramuscular Use.
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
Rx Only
NOVAPLUS®

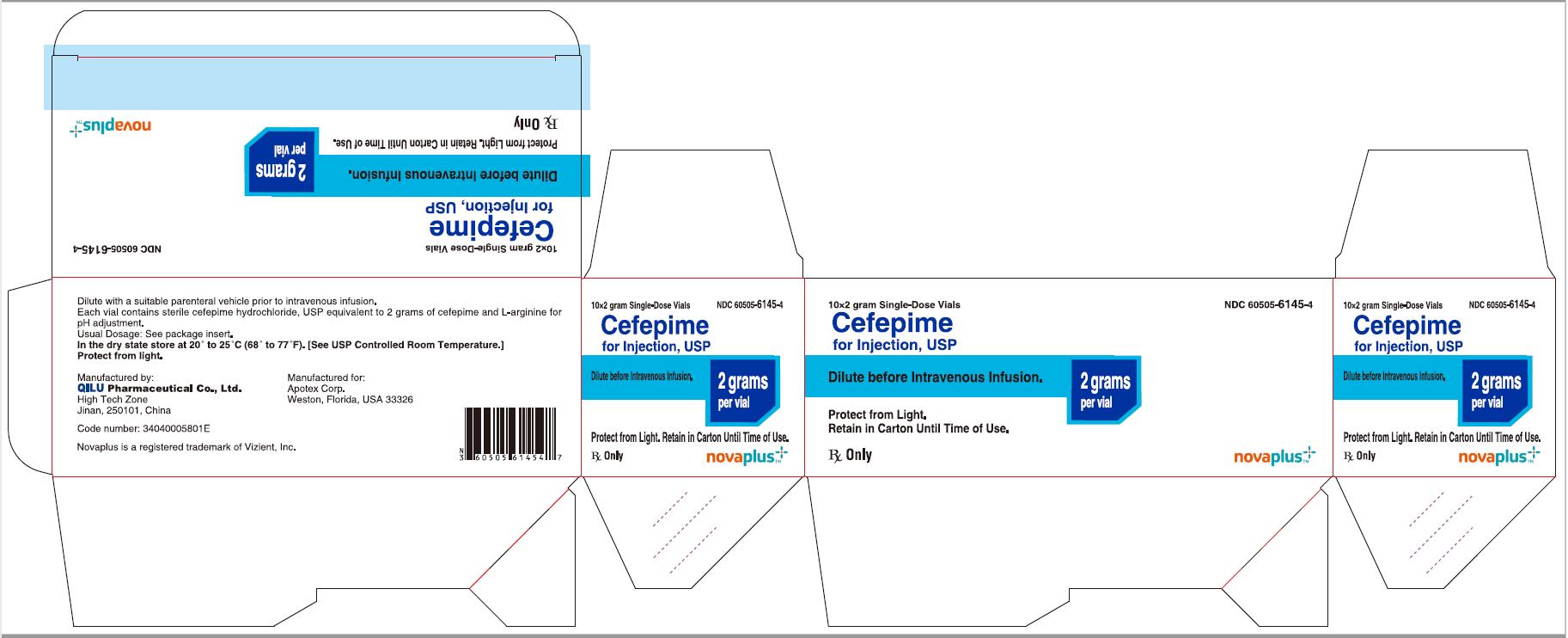
PRINCIPAL DISPLAY PANEL
10*2 gram Single-Dose Vials NDC 60505-6145-4
Cefepime for Injection, USP
2 grams per vial
Dilute before Intravenous Infusion.
Protect from Light. Retain in Carton Until Time of Use.
Rx Only
NOVAPLUS®