NDC Code(s) : 57664-047-88, 57664-046-88, 57664-048-88, 57664-049-88, 57664-050-88, 57664-051-88, 57664-052-88, 57664-083-88, 57664-084-88, 57664-085-88, 57664-086-88, 57664-087-88, 57664-088-88
Packager : Sun Pharmaceutical Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE CAPSULE | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LISDEXAMFETAMINE DIMESYLATELISDEXAMFETAMINE DIMESYLATE TABLET, CHEWABLE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ohm Laboratories Inc. | 184769029 | manufacture(57664-083, 57664-084, 57664-085, 57664-086, 57664-087, 57664-088, 57664-046, 57664-047, 57664-048, 57664-049, 57664-050, 57664-051, 57664-052) | |
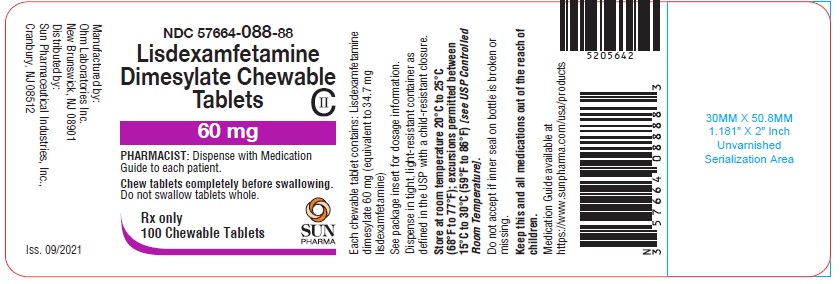
PRINCIPAL DISPLAY PANEL
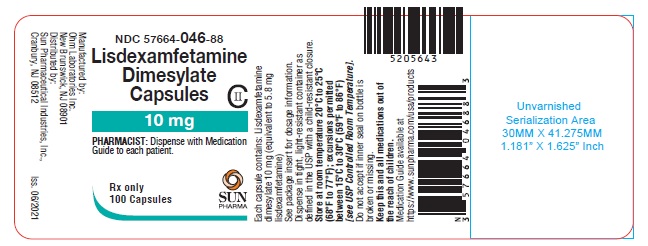
PRINCIPAL DISPLAY PANEL
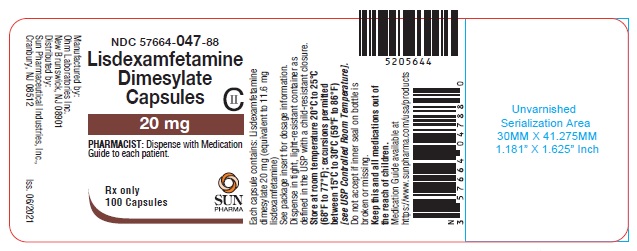
PRINCIPAL DISPLAY PANEL
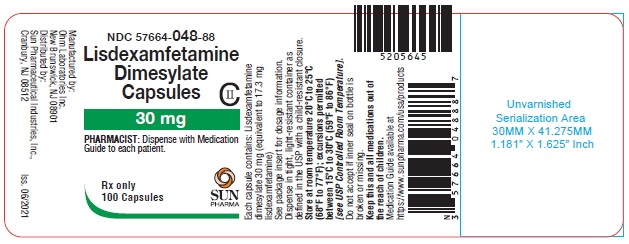
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL
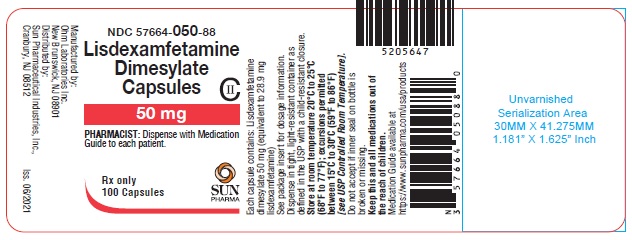
PRINCIPAL DISPLAY PANEL
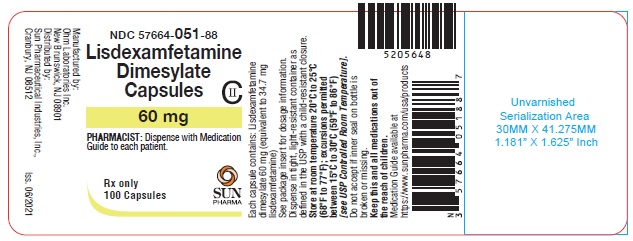
PRINCIPAL DISPLAY PANEL
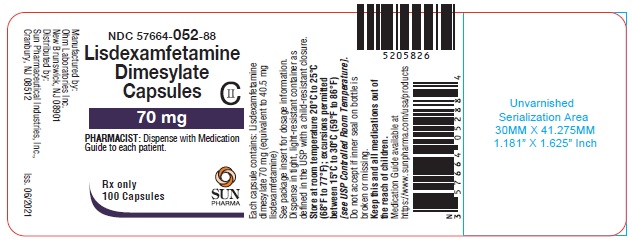
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL
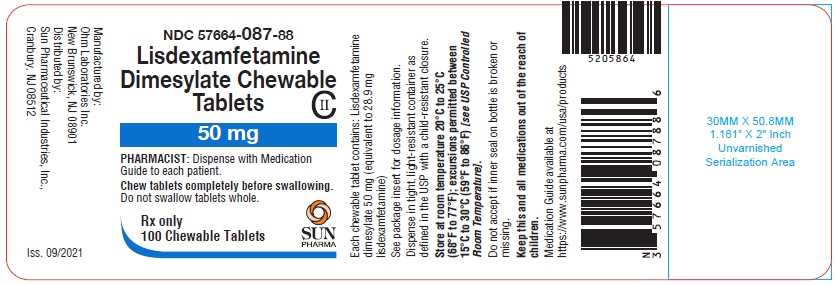
PRINCIPAL DISPLAY PANEL