NDC Code(s) : 55154-5764-9, 55154-5764-3, 55154-5765-9, 55154-5765-3, 55154-5762-0, 55154-5762-6, 55154-5762-4
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Trazodone HydrochlorideTrazodone Hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Trazodone HydrochlorideTrazodone Hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Trazodone HydrochlorideTrazodone Hydrochloride TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets USP
150 mg
10 Tablets

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablet, USP
150 mg

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
150 mg
100 Tablets

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
50 mg

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
50 mg
28 Tablets
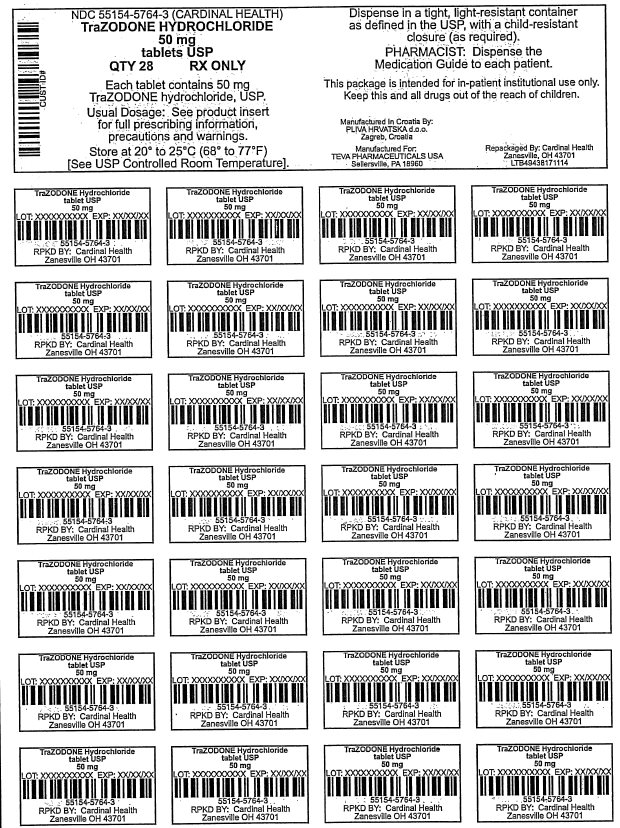
PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
50 mg

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
50 mg
30 Tablets

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride
100 mg
tablets USP

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
100 mg
28 Tablets
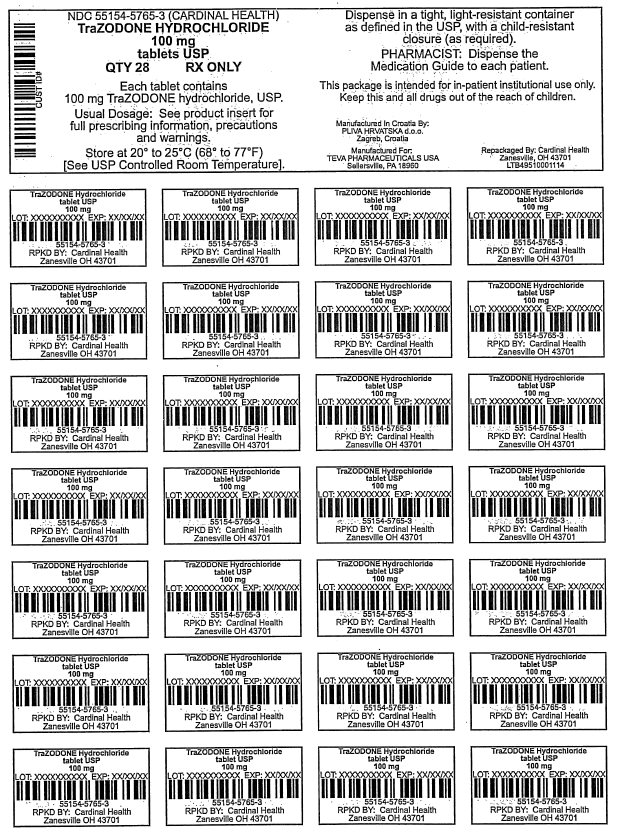
PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride
100 mg
tablets USP

PRINCIPAL DISPLAY PANEL
Trazodone Hydrochloride Tablets, USP
100 mg
30 Tablets










