NDC Code(s) : 55154-0975-6, 55154-0975-4, 55154-0975-0
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PROVIGILmodafinil TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
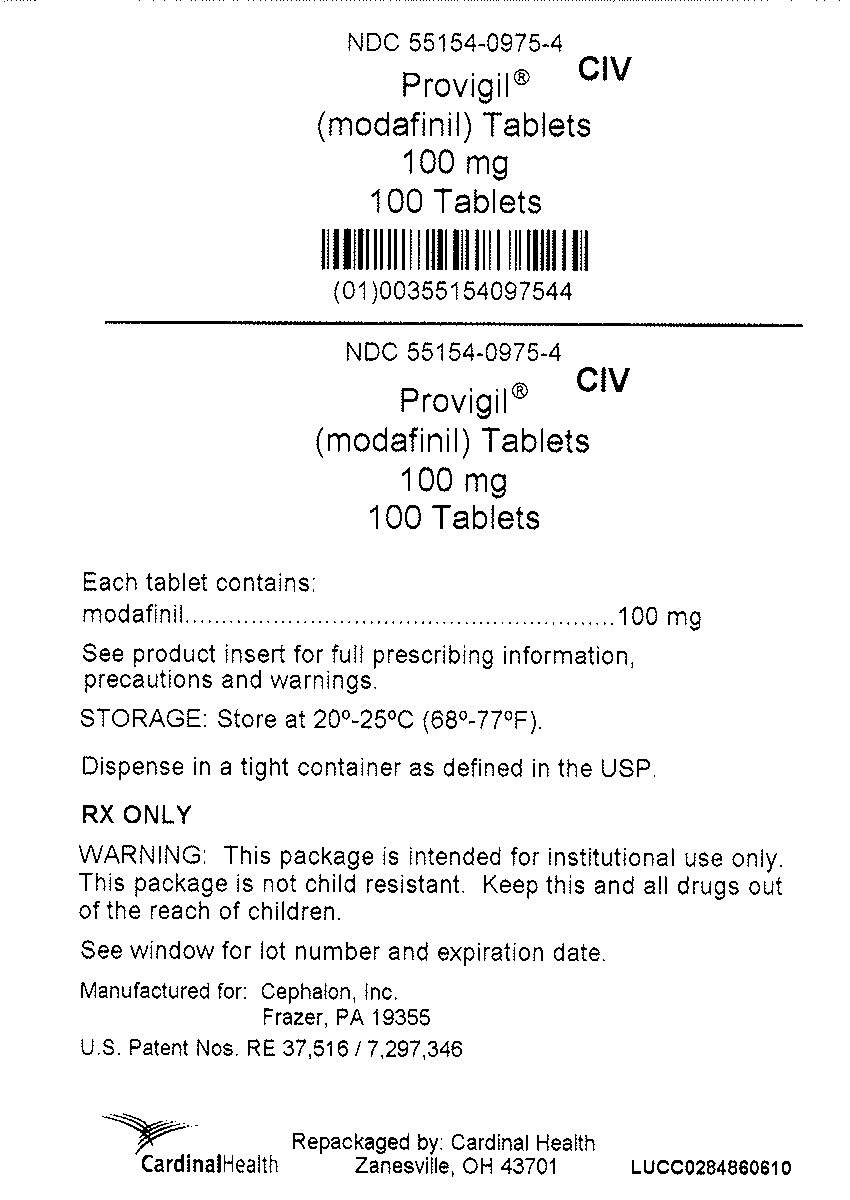
PRINCIPAL DISPLAY PANEL
NDC: 55154-0975-4
CIV
Provigil®
(modafinil) Tablets
100 mg
100 Tablets
Each tablet contains: modafinil 100 mg
See product insert for full prescribing information, precautions and warnings.
STORAGE: Store at 20°-25°C (68°-77°F).
Dispense in a tight container as defined in the USP.
RX ONLY
WARNING: This package is intended for institutional use only. This package is not child resistant. Keep this and all drugs out of the reach of children.
See window for lot number and expiration date.
Manufactured for: Cephalon, Inc.
Frazer, PA 19355
US Patent Nos. RE 37,516/ 7,297,346
Repackaged by: Cardinal Health
Zanesville, OH 43701
LUCC0284860610
PRINCIPAL DISPLAY PANEL
Provigil®
(modafinil) Tablet
CIV
100 mg

PRINCIPAL DISPLAY PANEL
Provigil® 100 mg
Modafinil
10 Tablets










