NDC Code(s) : 55150-237-01, 55150-238-05, 55150-239-30
Packager : Eugia US LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DEXAMETHASONE SODIUM PHOSPHATE DEXAMETHASONE SODIUM PHOSPHATE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXAMETHASONE SODIUM PHOSPHATE DEXAMETHASONE SODIUM PHOSPHATE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXAMETHASONE SODIUM PHOSPHATE DEXAMETHASONE SODIUM PHOSPHATE INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Eugia US LLC(968961354) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Eugia Pharma Specialities Limited | 650498244 | ANALYSIS(55150-237, 55150-238, 55150-239), MANUFACTURE(55150-237, 55150-238, 55150-239), PACK(55150-237, 55150-238, 55150-239) | |
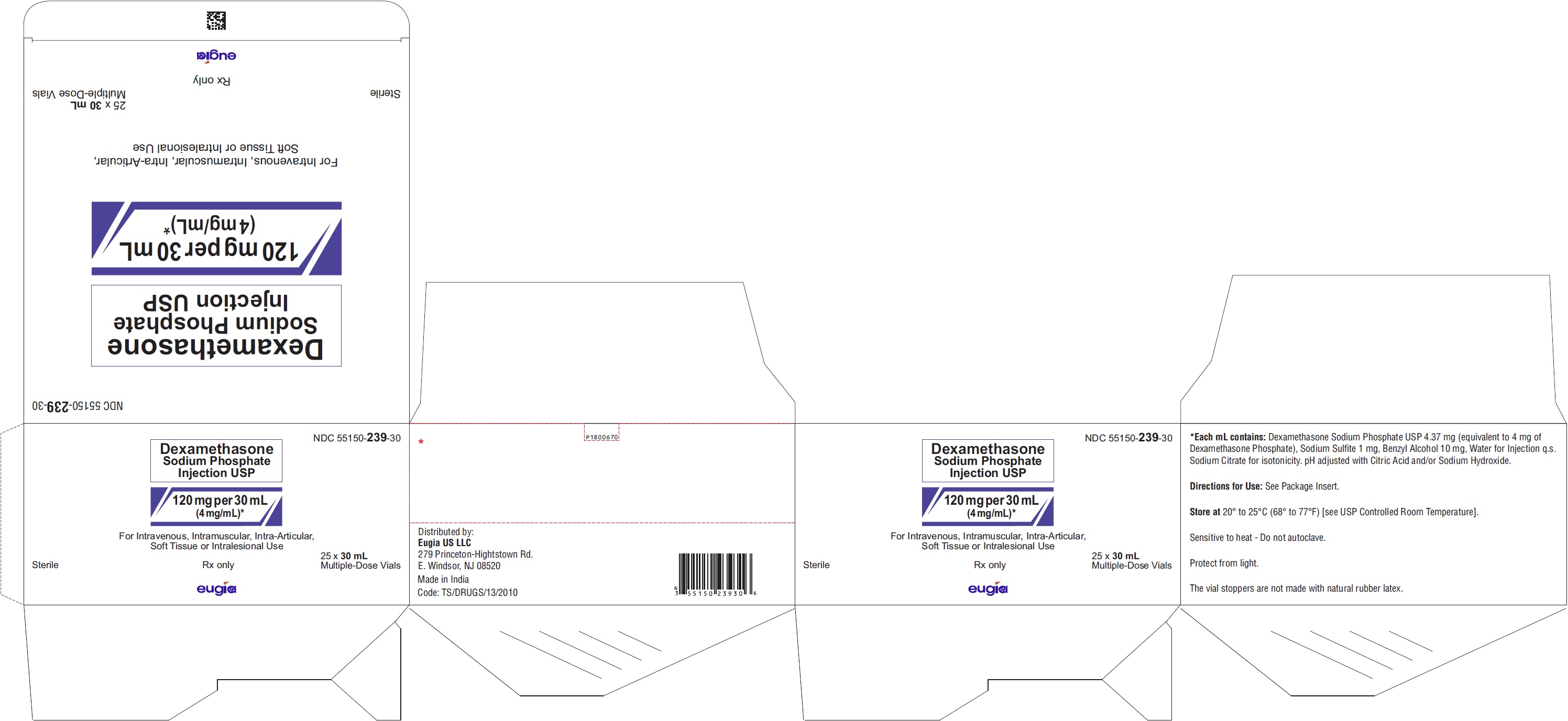
PRINCIPAL DISPLAY PANEL
NDC 55150-237-01
Dexamethasone
Sodium Phosphate
Injection USP
4 mg per mL
For I.V. or I.M. Use#
1 mL Single-Dose Vial
Rx only
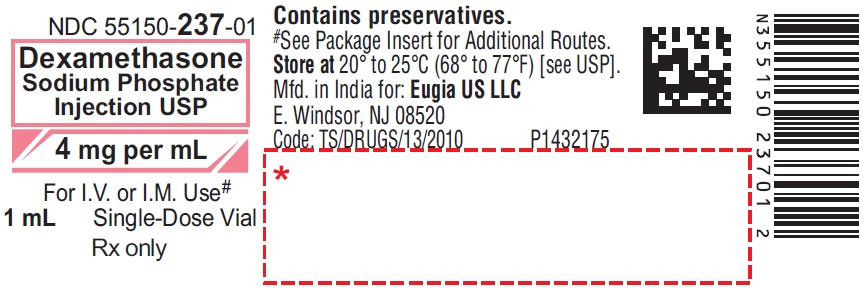
PRINCIPAL DISPLAY PANEL
NDC 55150-237-01
Dexamethasone
Sodium Phosphate
Injection USP
4 mg per mL*
For Intravenous, Intramuscular, Intra-Articular,
Soft Tissue or Intralesional Use
Sterile 25 x 1 mL
Single-Dose Vials
Rx only
eugia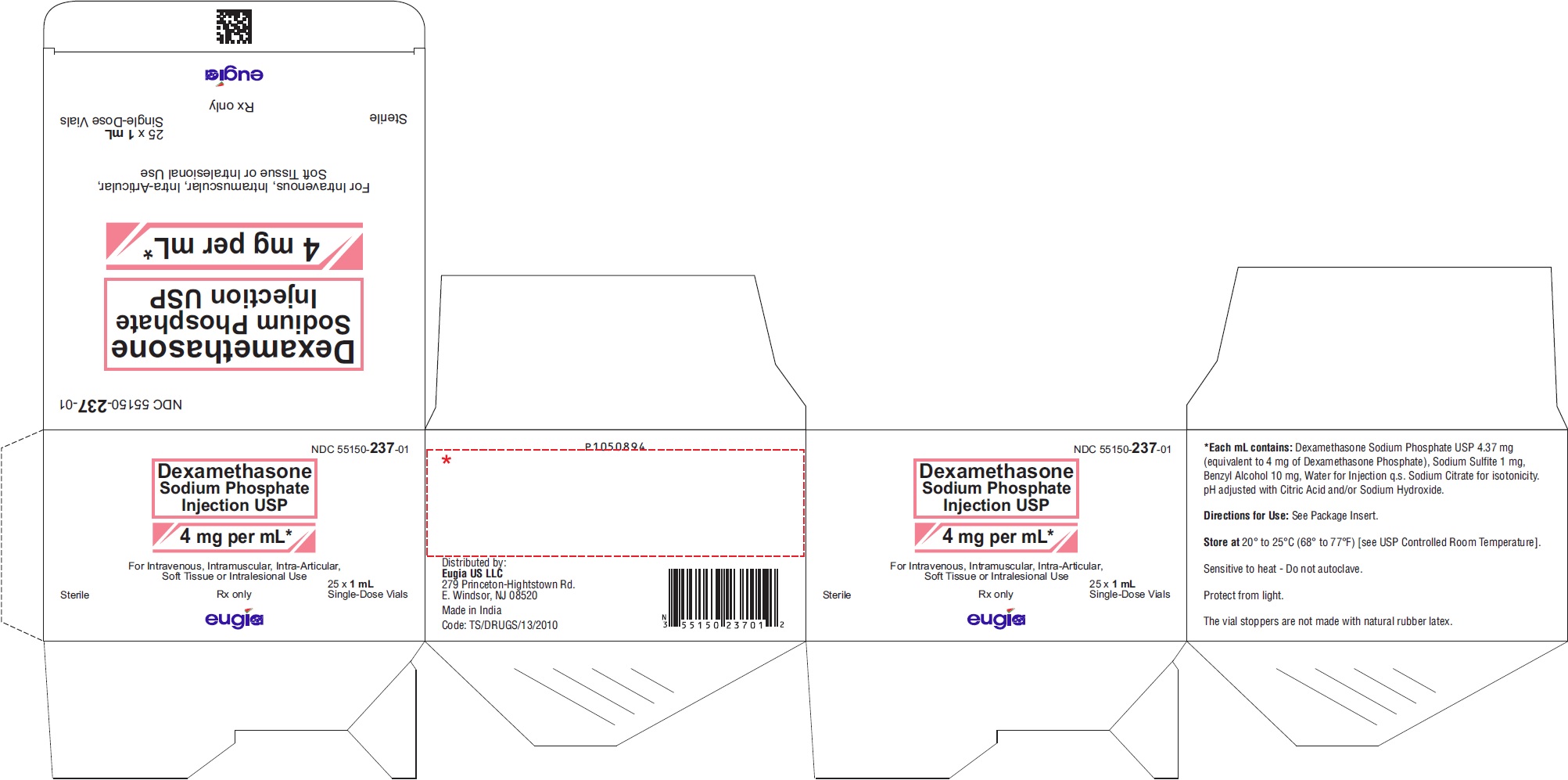
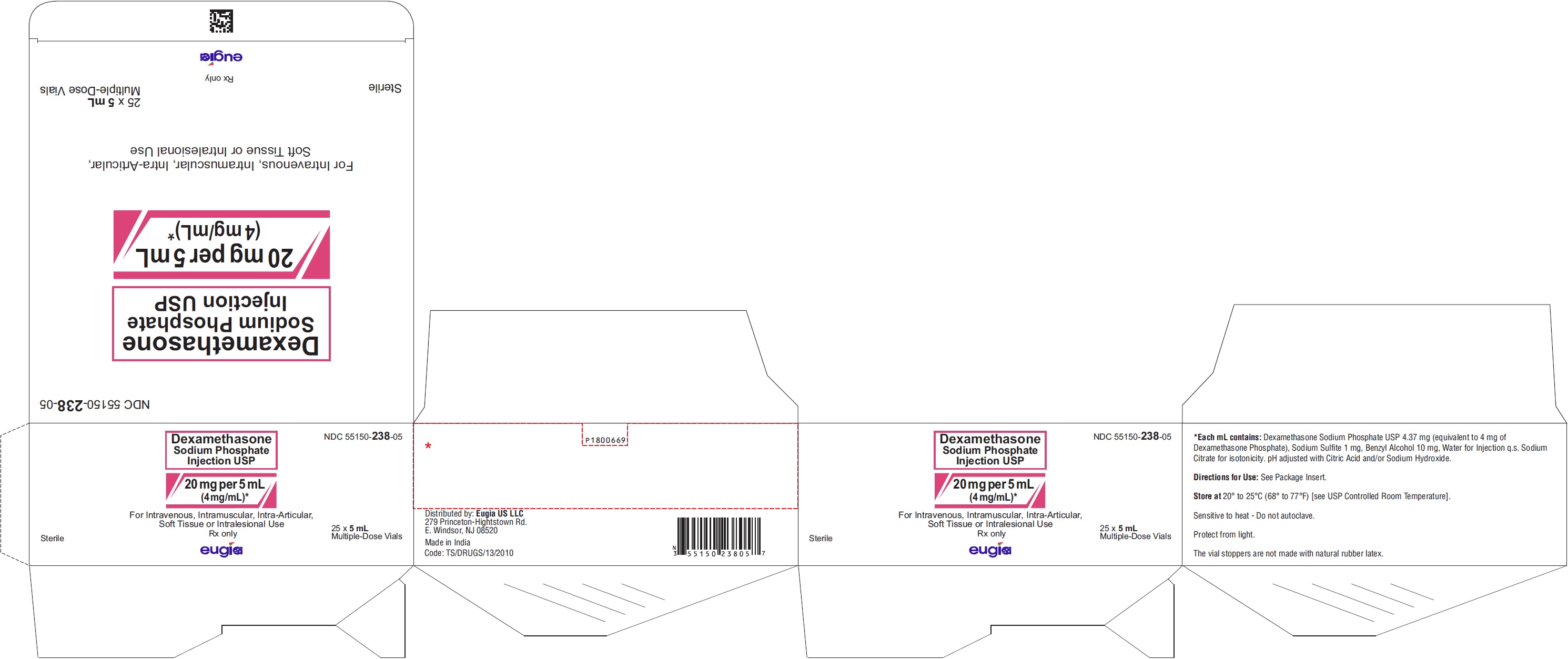
PRINCIPAL DISPLAY PANEL
NDC 55150-238-05
Dexamethasone
Sodium Phosphate
Injection USP
20 mg per 5 mL
(4 mg/mL)*
For I.V. or I.M. Use#
5 mL Multiple-Dose Vial
Rx only
PRINCIPAL DISPLAY PANEL
NDC 55150-238-05
Dexamethasone
Sodium Phosphate
Injection USP
20 mg per 5 mL
(4 mg/mL)*
For Intravenous, Intramuscular, Intra-Articular,
Soft Tissue or Intralesional Use
Sterile 25 x 5 mL
Multiple-Dose Vials
Rx only
eugia
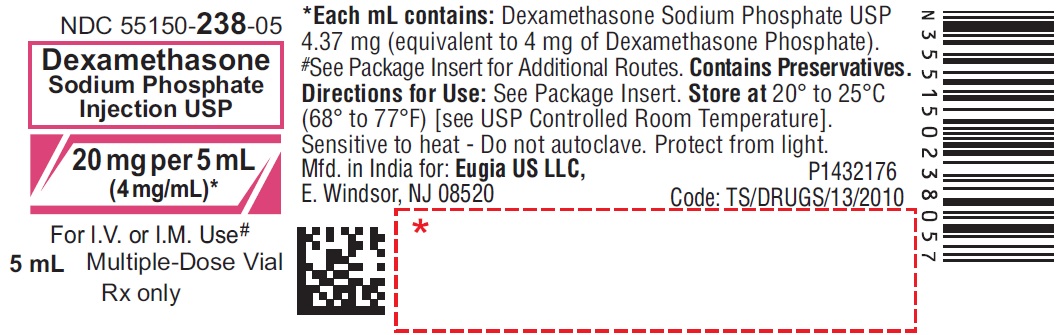
PRINCIPAL DISPLAY PANEL
NDC 55150-239-30
Dexamethasone
Sodium Phosphate
Injection USP
120 mg per 30 mL
(4 mg/mL)*
For I.V., I.M., Intra-Articular,
Soft Tissue or Intralesional Use
30 mL Multiple-Dose Vial
Rx only

PRINCIPAL DISPLAY PANEL
NDC 55150-239-30
Dexamethasone
Sodium Phosphate
Injection USP
120 mg per 30 mL
(4 mg/mL)*
For Intravenous, Intramuscular, Intra-Articular,
Soft Tissue or Intralesional Use
Sterile 25 x 30 mL
Multiple-Dose Vials
Rx only
eugia