NDC Code(s) : 50474-920-90, 50474-921-05
Packager : UCB, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Xyzallevocetirizine dihydrochloride TABLET, FILM COATED | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Xyzallevocetirizine dihydrochloride SOLUTION | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
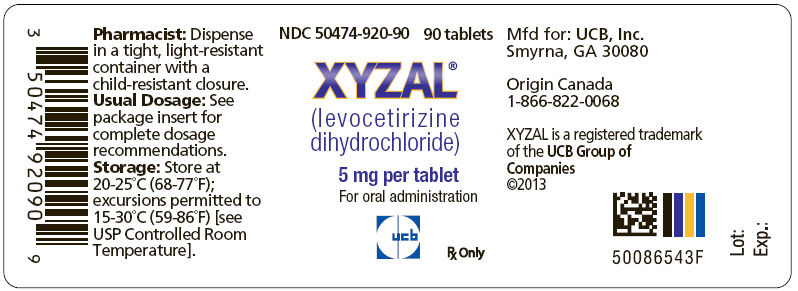
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
NDC 50474-920-90
90 tablets
XYZAL
®
(levocetirizine
dihydrochloride)
5 mg per tablet
For oral administration
ucb
Rx Only
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 148 mL Bottle Carton
NDC 50474-921-05
1 x 5 oz. (148 mL)
polypropylene bottle
XYZAL®
(levocetirizine dihydrochloride)
oral solution
2.5 mg/5 mL (0.5 mg/mL)
Rx Only
ucb










